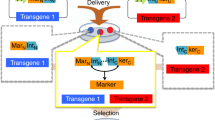
Abstract
Chromosomal deletions, as a genetic tool for functional genomics, remain underexploited for vertebrate stem cells mostly because presently available methods are too labor-intensive. To address this, we developed and validated a set of complementary retroviruses that creates a wide range of nested chromosomal deletions. When applied to mouse embryonic stem cells (ESCs), this retrovirus-based method yielded deletions ranging from 6 kb to 23 Mb (average 2.9 Mb), with an efficiency of 64% for drug-selected clones. Notably, several of the engineered ESC clones, mostly those with large deletions, showed major alteration in cell fate. In comparison to other methods that have also exploited retroviruses for chromosomal engineering, this modified strategy is more efficient and versatile because it bypasses the need for homologous recombination, and thus can be exploited for rapid and extensive functional screens in embryonic and adult stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Su, H., Wang, X. & Bradley, A. Nested chromosomal deletions induced with retroviral vectors in mice. Nat. Genet. 24, 92–95 (2000).
Yu, Y. & Bradley, A. Engineering chromosomal rearrangements in mice. Nat. Rev. Genet. 2, 780–790 (2001).
Liu, P., Jenkins, N.A. & Copeland, N.G. Efficient Cre-loxP-induced mitotic recombination in mouse embryonic stem cells. Nat. Genet. 30, 66–72 (2002).
Cervantes, R.B., Stringer, J.R., Shao, C., Tischfield, J.A. & Stambrook, P.J. Embryonic stem cells and somatic cells differ in mutation frequency and type. Proc. Natl. Acad. Sci. USA 99, 3586–3590 (2002).
Liu, X. et al. Trisomy eight in ES cells is a common potential problem in gene targeting and interferes with germ line transmission. Dev. Dyn. 209, 85–91 (1997).
Bradley, A. & van der Weyden, L. Mouse chromosome engineering for modeling human disease. Annu. Rev. Genomics Hum. Genet. 7, 247–276 (2006).
Brault, V., Pereira, P., Duchon, A. & Herault, Y. Modeling chromosomes in mouse to explore the function of genes, genomic disorders, and chromosomal organization. PLoS Genet. 2, e86 (2006).
Adams, D.J. et al. Mutagenic insertion and chromosome engineering resource (MICER). Nat. Genet. 36, 867–871 (2004).
De-Zolt, S. et al. High-throughput trapping of secretory pathway genes in mouse embryonic stem cells. Nucleic Acids Res. 34, e25 (2006).
Nord, A.S. et al. The International Gene Trap Consortium Website: a portal to all publicly available gene trap cell lines in mouse. Nucleic Acids Res. 34, D642–D648 (2006).
Chen, Y. et al. Genotype-based screen for ENU-induced mutations in mouse embryonic stem cells. Nat. Genet. 24, 314–317 (2000).
Lefebvre, L., Dionne, N., Karaskova, J., Squire, J.A. & Nagy, A. Selection for transgene homozygosity in embryonic stem cells results in extensive loss of heterozygosity. Nat. Genet. 27, 257–258 (2001).
Tybulewicz, V.L., Crawford, C.E., Jackson, P.K., Bronson, R.T. & Mulligan, R.C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153–1163 (1991).
Brummelkamp, T.R., Bernards, R. & Agami, R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2, 243–247 (2002).
Gaillard, C. & Strauss, F. Ethanol precipitation of DNA with linear polyacrylamide as carrier. Nucleic Acids Res. 18, 378 (1990).
Barnes, W.M. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc. Natl. Acad. Sci. USA 91, 2216–2220 (1994).
Kent, W.J. BLAT–the BLAST-like alignment tool. Genome Res. 12, 656–664 (2002).
Karolchik, D. et al. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 32, D493–D496 (2004).
Hubbard, T. et al. Ensembl 2005. Nucleic Acids Res. 33, D447–D453 (2005).
Margolin, A.A. et al. CGHAnalyzer: a stand-alone software package for cancer genome analysis using array-based DNA copy number data. Bioinformatics 21, 3308–3311 (2005).
Acknowledgements
We thank P. Chartrand, M. Therrien and colleagues for critically reading the manuscript; V. Paradis, S. Harton and E. Milot from the transgenic facility of IRIC; J. Cowell, M. Rossi and D. McQuaid for the aCGH service (Roswell Park Cancer Institute); J.-P. Laverdure from the bioinformatic service of IRIC;C. Charbonneau from the imaging service of IRIC; N. Fradet, M. Fréchette, A. Fredette, E. St-Hilaire, T. MacRae, C. Rondeau and P. Lussier for technical assistance; A. Nagy for providing the R1 ESCs and the pCX-EYFP construct (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto); A. Bradley for the pOG231 construct (Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton); R.G. Hawley for the MSCV vectors (The George Washington University Medical Center); M. van Lohuizen for the pRETRO-SUPER construct (The Netherlands Cancer Institute) and R. Jaenisch for the DR-4 mouse strain (Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology). This work was mostly supported by a grant from Génome Québec to G.S. and in part by a grant from the Réseau de Recherche en Transgenèse du Québec to J.H. M.B. is a recipient of a Canadian Institutes of Health Research (CIHR) studentship, and G.S. is a recipient of a Canada Research Chair in molecular genetics of stem cells and a scholar of the Leukemia Lymphoma Society of America.
Author information
Authors and Affiliations
Contributions
M.B. performed all the experiments and the analyses described herein, except for I-PCR (S.G.), spectral karyotyping and FISH analyses (J.H.). aCGH experiments and chimeras production were conducted by the services mentioned above. M.B. wrote the manuscript, prepared all the figures and performed the experiments under the guidance of G.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Generation of retroviral vectors. (PDF 38 kb)
Supplementary Fig. 2
Cre-induced recombination between integrated proviruses. (PDF 252 kb)
Supplementary Fig. 3
Display showing the chromosomal deletions confirmed in ES cells. (PDF 94 kb)
Supplementary Fig. 4
Evaluation of inter-chromosomal recombination events. (PDF 180 kb)
Supplementary Fig. 5
Full-length gels and blots. (PDF 161 kb)
Supplementary Table 1
Summary of the Cre-mediated recombination around 11 randomly chosen loci. (PDF 56 kb)
Supplementary Table 2
In vitro differentiation of primary and tertiary clones carrying deletions. (PDF 8 kb)
Rights and permissions
About this article
Cite this article
Bilodeau, M., Girard, S., Hébert, J. et al. A retroviral strategy that efficiently creates chromosomal deletions in mammalian cells. Nat Methods 4, 263–268 (2007). https://doi.org/10.1038/nmeth1011
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth1011
This article is cited by
-
The Human Endogenous Retrovirus Link between Genes and Environment in Multiple Sclerosis and in Multifactorial Diseases Associating Neuroinflammation
Clinical Reviews in Allergy & Immunology (2010)
-
A transposon-based chromosomal engineering method to survey a large cis-regulatory landscape in mice
Nature Genetics (2009)