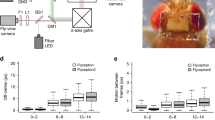
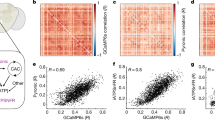
Abstract
Genetically encoded calcium sensors have enabled monitoring of neural activity in vivo using optical imaging techniques. Linking neural activity to complex behavior remains challenging, however, as most imaging systems require tethering the animal, which can impact the animal's behavioral repertoire. Here, we report a method for monitoring the brain activity of untethered, freely walking Drosophila melanogaster during sensorially and socially evoked behaviors to facilitate the study of neural mechanisms that underlie naturalistic behaviors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kohatsu, S. & Yamamoto, D. Nat. Commun. 6, 6457 (2015).
Taylor, G.J. et al. J. Exp. Biol. 218, 3118–3127 (2015).
Flusberg, B.A. et al. Nat. Methods 5, 935–938 (2008).
Zheng, M., Cao, P., Yang, J., Xu, X.Z.S. & Feng, Z. J. Neurosci. Methods 206, 78–82 (2012).
Nguyen, J.P. et al. Proc. Natl. Acad. Sci. USA 113, E1074–E1081 (2015).
Fiala, A. et al. Curr. Biol. 12, 1877–1884 (2002).
Venken, K.J.T., Simpson, J.H. & Bellen, H.J. Neuron 72, 202–230 (2011).
Sokolowski, M.B. Nat. Rev. Genet. 2, 879–890 (2001).
Shih, C.-T. et al. Curr. Biol. 25, 1249–1258 (2015).
Strausfeld, N.J. & Hirth, F. Science 340, 157–161 (2013).
Seelig, J.D. et al. Nat. Methods 7, 535–540 (2010).
van Swinderen, B. & Greenspan, R.J. Nat. Neurosci. 6, 579–586 (2003).
Kohatsu, S., Koganezawa, M. & Yamamoto, D. Neuron 69, 498–508 (2011).
Clowney, E.J., Iguchi, S., Bussell, J.J., Scheer, E. & Ruta, V. Neuron 87, 1036–1049 (2015).
Lin, Y.-Y. et al. Biomed. Opt. Express 6, 514–523 (2015).
Bath, D.E. et al. Nat. Methods 11, 756–762 (2014).
Wu, M.-C. et al. Proc. Natl. Acad. Sci. USA 111, 5367–5372 (2014).
Stocker, R.F., Heimbeck, G., Gendre, N. & de Belle, J.S. J. Neurobiol. 32, 443–456 (1997).
Yamamoto, D. & Koganezawa, M. Nat. Rev. Neurosci. 14, 681–692 (2013).
Hoopfer, E.D., Jung, Y., Inagaki, H.K., Rubin, G.M. & Anderson, D.J. eLife 4, e11346 (2015).
Edelstein, A., Amodaj, N., Hoover, K., Vale, R. & Stuurman, N. Curr. Protoc. Mol. Biol. 94, 14.20.1–14.20.17 (2010).
Kalman, R.E. J. Basic Eng. 82, 35–45 (1960).
Bradski, G. Doctor Dobb's Journal 25, 120–126 (2000).
Dagum, L. & Menon, R. IEEE Computational Science & Engineering 5, 46–55 (1998).
Straw, A.D. & Dickinson, M.H. Source Code Biol. Med. 4, 5 (2009).
Pau, G., Fuchs, F., Sklyar, O., Boutros, M. & Huber, W. Bioinformatics 26, 979–981 (2010).
Acknowledgements
We thank F. Cardone, I. Freerksen, and K. Katsuki for assistance with designing and fabricating electronic and mechanical components; J. Clayden for assistance with image registration; and Z. Liu for advice on optics. We would like to thank D. Anderson (Caltech, Pasadena, CA) for the P1–Gal4 fly strain. We also thank members of the Greenspan and Stevens laboratories for comments on the manuscript and helpful advice during the course of the project. Confocal microscopy experiments used equipment that is funded by the UCSD School of Medicine Microscopy Core Grant P30 NS047101. This work was supported by the Kavli Institute for Brain and Mind Innovative Research Grant 2014-010 (T.K.) and by the Qualcomm Institute's Calit2 Strategic Research Opportunities Program CITS145 (R.J.G. and D.G.).
Author information
Authors and Affiliations
Contributions
D.G. and T.K., conception and design, development of the hardware and software, acquisition of data, analysis and interpretation of data, and drafting or revising the article; R.J.G., conception and design and drafting or revising the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Flyception system overview
(a) Flyception system components, 1) Fly arena with delrin diffuser backplate, 2) Far-red backlight, 3) Two-axis galvanometer mirror assembly, 4) 580 nm longpass dichroic mirror, 5) 488 nm longpass dichroic mirror, 6 and 7) Mirrors to reflect laser beam, 8) 2X Laser beam expander, 9) 473 nm DPSS laser, 10) Fly-view camera and lens, 11) Fluorescence-view EM-CCD camera and lens, 12) Arena view camera and lens with red longpass filter, 13) Flash, 14 and 15) Optical filter housing assembly, 16) Arduino Mega 2560 for triggering arena, fly and fluorescence view cameras, and flash, 17) Backlight power supply, 18) Laser power supply, 19, 20 and 21) XYZ translational stage. Scale bar, 1 in, is the distance between consecutive holes on the breadboard. (b) A view of the primary (1) and secondary (2) galvanometer mirrors and the imaging arena. Lines shown in red, green and dotted blue indicate light paths for the red backlight, green fluorescence and blue laser illumination, with blue laser beam directed towards the arena and red and green wavelength lights emanating away from the arena. The red backlight passes through the 580 nm longpass dichroic mirror (3) to the fly-view camera, while shorter blue and green wavelengths are reflected. The blue laser is reflected off the 488 nm dichroic mirror (4), while green fluorescence passes through to the fluorescence-view camera. (c) Schematic of concave arena surface maintaining fly head focus and body axis orthogonality irrespective of fly position in arena and galvanometer mirror rotation. (d) Rendering of fly arena fixture.
Supplementary Figure 2 Fly-view tracking outline
Algorithmic steps involved in detecting the fly head center in every fly-view image frame. (a) Raw fly-view image, (b) fly body silhouette, (c) fly contour outline, (d) convex hull estimate of fly contour, (e) body axis estimate of fly, (f) extreme fly body edge point estimate, (g) manual offset from top-of-head estimated point, (h) turn galvanometer mirrors to compensate for distance between estimated head point and center of image. Scale bar, 200 μm.
Supplementary Figure 3 Post-acquisition image processing pipeline
(a) Components and flow of flyception image processing pipeline. (b) Raw fly-view image. (c) A binary image of the fly after adaptive thresholding. (d) An extracted center region. (e) A binary image of the fly after global thresholding. (f) Intersection of (c) and (e). (g) After noise removal with an opening morphological operation. (h) A window mask after size filtering. (i) An overlaid image of (i) and (b). (j) A fly-view image in which a flash (strobe) was detected. (k) A fluo-view frame of the same instance as (j). (l) An overlaid image of (j) and (k) after template matching. (m) An overlaid image of fly-view, a window mask (black), and fluo-view (green) from the same instance. (n) LoG image of (b). (o) An example of an out-of-focus image. (p) An example of a tilted head. Scale bar, 200 μm.
Supplementary Figure 4 Raw GCaMP6s fluorescence images during sensory- and socially-evoked behavior
(a-d) Raw GCaMP6s fluorescence images at the time points indicated with the open and black arrowheads in Fig. 2b, Fig. 3c, Supplementary Fig. 6c, and Fig. 3f, respectively. Heat map representations of the corresponding frames are shown in Fig. 2c, Fig. 3b, Supplementary Fig. 6b, and Fig. 3e. See Supplementary Table 1 for detailed genotypes. Scale bar, 200 μm.
Supplementary Figure 5 Localization of Fru-positive neurons
(a) A confocal fluorescence image and (b) fluo-view image of a male brain that expresses myr-EGFP under the driver fru-Gal4 viewed through the imaging window. (c) A fluo-view of a male brain expressing GCaMP6s under fru-Gal4. Orange arrowheads indicate the dorsal posterior brain regions that include P1 clusters. Scale bar, 200 μm.
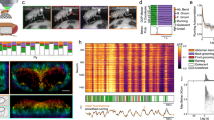
Supplementary Figure 6 Activity increase in fruitless-expressing neurons during naturally evoked courtship without physical contact between flies
(a) Arena-view of a male fly with fru-Gal4 and UAS-GCaMP6sattP2 transgenes courting a female of the same genotype. The inset in (a) shows the corresponding fly-view frame in which the distance between the flies was the shortest before an activity increase was detected. Arrowheads indicate tips of tarsi. Scale bar, 10 mm (200 μm in insets). (b) Changes of fluorescence intensity in the male brain before and after the male approaches the female. The heat map of dF/F images is overlaid with fly-view images of the corresponding frames. See Supplementary Fig. 4c for raw fluorescence images. Scale bar, 200 μm. (c) Time series of dF/F values - loess curve fit (blue) with 95% confidence interval band (gray), and distance between flies (black). The open arrowheads and black arrowheads indicate the time points at which the left and right images in (b) were captured, respectively. The grey arrowheads indicate the time point for (a).
Supplementary Figure 7 Fluo-view image of the resolution test target used for system calibration
Image of NBS 1952 Resolution Test Target captured with fluo-view camera. Numbers in the image indicate line pairs per millimeter (lp mm-1). A crosshair is 610 µm in length and width and two concentric circles are 250 µm and 500 µm in diameter.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1 and 2 (PDF 1413 kb)
Supplementary Software
Flyception source code, analysis scripts, and instructions. (ZIP 2218 kb)
Flyception accurately tracks a freely walking fly.
A fly with an imaging window was being tracked at 1000 Hz update rate. Left, raw fly-view image, right, the result of post-hoc image registration. (MOV 2201 kb)
GCaMP6s responses of olfactory projection neurons to ethanol odor.
GCaMP6s was expressed in olfactory projection neurons under the control of GH146-Gal4. The odor port opens at 1 s into the video and a 2 s pulse of ethanol odor was given. (MOV 1157 kb)
Activity of Fru-expressing neurons increased at a contact with a female.
GCaMP6s was expressed under the control of fru-Gal4. Flies contact at around 8 s into the video. (MOV 807 kb)
Activity of Fru-expressing neurons increased without a contact with a female.
GCaMP6s was expressed under the control of fru-Gal4. Note that at around 3 s into the video a male tarsus comes close to a female tarsus but does not touch. (MOV 827 kb)
Activity of P1-interneurons increased during courtship with a female.
GCaMP6s was expressed under the control of P1-Gal4. Flies contact at around 2 s into the video. (MOV 2950 kb)
Rights and permissions
About this article
Cite this article
Grover, D., Katsuki, T. & Greenspan, R. Flyception: imaging brain activity in freely walking fruit flies. Nat Methods 13, 569–572 (2016). https://doi.org/10.1038/nmeth.3866
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3866
This article is cited by
-
Parallelized computational 3D video microscopy of freely moving organisms at multiple gigapixels per second
Nature Photonics (2023)
-
Imaging brain activity during complex social behaviors in Drosophila with Flyception2
Nature Communications (2020)
-
Recent Advances in the Genetic Dissection of Neural Circuits in Drosophila
Neuroscience Bulletin (2019)
-
Long-term optical brain imaging in live adult fruit flies
Nature Communications (2018)
-
Pan-neuronal calcium imaging with cellular resolution in freely swimming zebrafish
Nature Methods (2017)