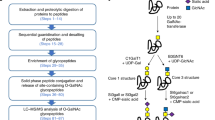
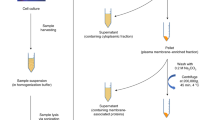
Abstract
Glycans have essential roles in biology and the etiology of many diseases. A major hurdle in studying glycans through functional glycomics is the lack of methods to release glycans from diverse types of biological samples. Here we describe an oxidative strategy using household bleach to release all types of free reducing N-glycans and O-glycan-acids from glycoproteins, and glycan nitriles from glycosphingolipids. Released glycans are directly useful in glycomic analyses and can be derivatized fluorescently for functional glycomics. This chemical method overcomes the limitations in glycan generation and promotes archiving and characterization of human and animal glycomes and their functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Paulson, J.C., Blixt, O. & Collins, B.E. Sweet spots in functional glycomics. Nat. Chem. Biol. 2, 238–248 (2006).
Smith, D.F., Song, X. & Cummings, R.D. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 480, 417–444 (2010).
Cummings, R.D. The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 5, 1087–1104 (2009).
Alvarez, R.A. & Blixt, O. Identification of ligand specificities for glycan-binding proteins using glycan arrays. Methods Enzymol. 415, 292–310 (2006).
Varki, A. et al. Essentials of Glycobiology 2nd edn. (Cold Spring Harbor Laboratory Press, 2009).
Royle, L. et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal. Biochem. 376, 1–12 (2008).
Doneanu, C.E., Chen, W. & Gebler, J.C. Analysis of oligosaccharides derived from heparin by ion-pair reversed-phase chromatography/mass spectrometry. Anal. Chem. 81, 3485–3499 (2009).
Ruhaak, L.R. et al. Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 397, 3457–3481 (2010).
Ly, M. et al. The proteoglycan bikunin has a defined sequence. Nat. Chem. Biol. 7, 827–833 (2011).
Stumpo, K.A. & Reinhold, V.N. The N-glycome of human plasma. J. Proteome Res. 9, 4823–4830 (2010).
Prien, J.M., Ashline, D.J., Lapadula, A.J., Zhang, H. & Reinhold, V.N. The high-mannose glycans from bovine ribonuclease B isomer characterization by ion trap MS. J. Am. Soc. Mass Spectrom. 20, 539–556 (2009).
Wada, Y. et al. Comparison of the methods for profiling glycoprotein glycans–HUPO Human Disease Glycomics/Proteome Initiative multi-institutional study. Glycobiology 17, 411–422 (2007).
Alvarez-Manilla, G. et al. Tools for glycomics: relative quantitation of glycans by isotopic permethylation using 13CH3I. Glycobiology 17, 677–687 (2007).
Xia, B., Feasley, C.L., Sachdev, G.P., Smith, D.F. & Cummings, R.D. Glycan reductive isotope labeling for quantitative glycomics. Anal. Biochem. 387, 162–170 (2009).
Zaia, J. Mass spectrometry and the emerging field of glycomics. Chem. Biol. 15, 881–892 (2008).
Stevens, J., Blixt, O., Paulson, J.C. & Wilson, I.A. Glycan microarray technologies: tools to survey host specificity of influenza viruses. Natl. Rev. 4, 857–864 (2006).
Song, X. et al. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem. Biol. 16, 36–47 (2009).
Rillahan, C.D. & Paulson, J.C. Glycan microarrays for decoding the glycome. Annu. Rev. Biochem. 80, 797–823 (2011).
de Boer, A.R., Hokke, C.H., Deelder, A.M. & Wuhrer, M. General microarray technique for immobilization and screening of natural glycans. Anal. Chem. 79, 8107–8113 (2007).
Blixt, O. et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. USA 101, 17033–17038 (2004).
Song, X. et al. Shotgun glycomics: a microarray strategy for functional glycomics. Nat. Methods 8, 85–90 (2011).
Zhu, X. & Schmidt, R.R. New principles for glycoside-bond formation. Angew. Chem. Int. Ed. 48, 1900–1934 (2009).
Seeberger, P.H. Automated carbohydrate synthesis to drive chemical glycomics. Chem. Commun. 10, 1115–1121 (2003).
Pozsgay, V. Recent developments in synthetic oligosaccharide-based bacterial vaccines. Curr. Top. Med. Chem. 8, 126–140 (2008).
Murata, T. & Usui, T. Enzymatic synthesis of oligosaccharides and neoglycoconjugates. Biosci. Biotechnol. Biochem. 70, 1049–1059 (2006).
Kanemitsu, T. & Kanie, O. Recent developments in oligosaccharide synthesis: tactics, solid-phase synthesis and library synthesis. Comb. Chem. High Throughput Screen. 5, 339–360 (2002).
Xia, B. et al. Versatile fluorescent derivatization of glycans for glycomic analysis. Nat. Methods 2, 845–850 (2005).
Song, X., Xia, B., Lasanajak, Y., Smith, D.F. & Cummings, R.D. Quantifiable fluorescent glycan microarrays. Glycoconj. J. 25, 15–25 (2008).
Song, X., Lasanajak, Y., Xia, B., Smith, D.F. & Cummings, R.D. Fluorescent glycosylamides produced by microscale derivatization of free glycans for natural glycan microarrays. ACS Chem. Biol. 4, 741–750 (2009).
Song, X. et al. Generation of a natural glycan microarray using 9-fluorenylmethyl chloroformate (FmocCl) as a cleavable fluorescent tag. Anal. Biochem. 395, 151–160 (2009).
Yosizawa, Z., Sato, T. & Schmid, K. Hydrazinolysis of α-1-acid glycoprotein. Biochim. Biophys. Acta 121, 417–420 (1966).
Huang, Y., Mechref, Y. & Novotny, M.V. Microscale nonreductive release of O-linked glycans for subsequent analysis through MALDI mass spectrometry and capillary electrophoresis. Anal. Chem. 73, 6063–6069 (2001).
Ito, M. & Yamagata, T. A novel glycosphingolipid-degrading enzyme cleaves the linkage between the oligosaccharide and ceramide of neutral and acidic glycosphingolipids. J. Biol. Chem. 261, 14278–14282 (1986).
Ito, M. & Yamagata, T. Endoglycoceramidase from Rhodococcus species G-74–2. Methods Enzymol. 179, 488–496 (1989).
Plummer, T.H. Jr. & Tarentino, A.L. Purification of the oligosaccharide-cleaving enzymes of Flavobacterium meningosepticum. Glycobiology 1, 257–263 (1991).
Ashline, D.J. et al. Structural characterization by multistage mass spectrometry (MSn) of human milk glycans recognized by human rotaviruses. Mol. Cell. Proteomics 13, 2961–2974 (2014).
Hawkins, C.L. & Davies, M.J. Hypochlorite-induced damage to proteins: formation of nitrogen-centered radicals from lysine residues and their role in protein fragmentation. Biochem. J. 332, 617–625 (1998).
Pereira, W.E., Hoyano, Y., Summons, R.E., Bacon, V.A. & Duffield, A.M. Chlorination studies. II. Reaction of aqueous hypochlorous acid with α-amino acids and dipeptides. Biochim. Biophys. Acta, Gen. Subj. 313, 170–180 (1973).
Tretter, V., Altmann, F. & Marz, L. Peptide-N4-(N-acetyl-β-glucosaminyl)asparagine amidase F cannot release glycans with fucose attached α1→3 to the asparagine-linked N-acetylglucosamine residue. Eur. J. Biochem. 199, 647–652 (1991).
Guile, G.R. et al. Identification of highly fucosylated N-linked oligosaccharides from the human parotid gland. Eur. J. Biochem. 258, 623–656 (1998).
Wang, H. et al. Design and synthesis of glycoprotein-based multivalent glyco-ligands for influenza hemagglutinin and human galectin-3. Bioorg. Med. Chem. 21, 2037–2044 (2013).
Kajihara, Y. et al. Prompt chemoenzymatic synthesis of diverse complex-type oligosaccharides and its application to the solid-phase synthesis of a glycopeptide with Asn-linked sialyl-undeca- and asialo-nonasaccharides. Chemistry 10, 971–985 (2004).
Mine, Y. (ed.) Egg Bioscience and Biotechnology (John Wiley and Sons, Inc., 2008).
Yamamoto, K., Kadowaki, S., Watanabe, J. & Kumagai, H. Transglycosylation activity of Mucor hiemalis endo-β-N-acetylglucosaminidase which transfers complex oligosaccharides to the N-acetylglucosamine moieties of peptides. Biochem. Biophys. Res. Commun. 203, 244–252 (1994).
Lundborg, M. & Widmalm, G. Structural analysis of glycans by NMR chemical shift prediction. Anal. Chem. 83, 1514–1517 (2011).
Yu, Y. et al. Functional glycomic analysis of human milk glycans reveals the presence of virus receptors and embryonic stem cell biomarkers. J. Biol. Chem. 287, 44784–44799 (2012).
Byrd-Leotis, L. et al. Shotgun glycomics of pig lung identifies natural endogenous receptors for influenza viruses. Proc. Natl. Acad. Sci. USA 111, E2241–E2250 (2014).
Song, X. et al. Shotgun glycomics: a microarray strategy for functional glycomics. Nat. Methods 8, 85–90 (2011).
Yu, Y. et al. Human milk contains novel glycans that are potential decoy receptors for neonatal rotaviruses. Mol. Cell. Proteomics 13, 2944–2960 (2014).
Goetz, J.A., Novotny, M.V. & Mechref, Y. Enzymatic/chemical release of O-glycans allowing MS analysis at high sensitivity. Anal. Chem. 81, 9546–9552 (2009).
Sekiya, S., Wada, Y. & Tanaka, K. Derivatization for stabilizing sialic acids in MALDI-MS. Anal. Chem. 77, 4962–4968 (2005).
Wang, C., Fan, W., Zhang, P., Wang, Z. & Huang, L. One-pot nonreductive O-glycan release and labeling with 1-phenyl-3-methyl-5-pyrazolone followed by ESI-MS analysis. Proteomics 11, 4229–4242 (2011).
Schnaar, R.L. Isolation of glycosphingolipids. Methods Enzymol. 230, 348–370 (1994).
Reddy, C.R., Vijeender, K., Bhusan, P.B., Madhavi, P.P. & Chandrasekhar, S. Reductive N-alkylation of aromatic amines and nitro compounds with nitriles using polymethylhydrosiloxane. Tetrahedr. Lett. 48, 2765–2768 (2007).
Neogi, S. & Naskar, D. One-pot reductive mono-N-alkylation of aromatic nitro compounds using nitriles as alkylating reagents. Synth. Commun. 41, 1901–1915 (2011).
Sajiki, H., Ikawa, T. & Hirota, K. Reductive and catalytic monoalkylation of primary amines using nitriles as an alkylating reagent. Org. Lett. 6, 4977–4980 (2004).
Nacario, R., Kotakonda, S., Fouchard, D.M.D., Tillekeratne, L.M.V. & Hudson, R.A. Reductive monoalkylation of aromatic and aliphatic nitro compounds and the corresponding amines with nitriles. Org. Lett. 7, 471–474 (2005).
Ohno, N. et al. Solubilization of yeast cell-wall β-(1→3)-d-glucan by sodium hypochlorite oxidation and dimethyl sulfoxide extraction. Carbohydr. Res. 316, 161–172 (1999).
Rees, M.D., Hawkins, C.L. & Davies, M.J. Hypochlorite-mediated fragmentation of hyaluronan, chondroitin sulfates, and related N-acetyl glucosamines: evidence for chloramide intermediates, free radical transfer reactions, and site-specific fragmentation. J. Am. Chem. Soc. 125, 13719–13733 (2003).
Rees, M.D., Pattison, D.I. & Davies, M.J. Oxidation of heparan sulphate by hypochlorite: role of N-chloro derivatives and dichloramine-dependent fragmentation. Biochem. J. 391, 125–134 (2005).
Rees, M.D. & Davies, M.J. Heparan sulfate degradation via reductive homolysis of its N-chloro derivatives. J. Am. Chem. Soc. 128, 3085–3097 (2006).
Weiss, S.J., Lampert, M.B. & Test, S.T. Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science 222, 625–628 (1983).
Prütz, W.A. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch. Biochem. Biophys. 332, 110–120 (1996).
Bruggink, C. et al. Glycan profiling of urine, amniotic fluid and ascitic fluid from galactosialidosis patients reveals novel oligosaccharides with reducing end hexose and aldohexonic acid residues. FEBS J. 277, 2970–2986 (2010).
Anumula, K.R. & Taylor, P.B. A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal. Biochem. 203, 101–108 (1992).
Laval, G. & Golding, B.T. One-pot sequence for the decarboxylation of α-amino acids. Synlett 4, 542–546 (2003).
Acknowledgements
We thank J. Heimburg-Molinaro (Beth Israel Deaconess Medical Center) for help in scientific discussion and preparing the manuscript. We thank H. Sun (Wuhan University) for providing rAAL2 lectin. This work was supported by the US National Institutes of Health (EUREKA grant GM085448 to D.F.S., BTRC grant P41GM10369 to R.D.C. and Common Fund Glycoscience grant U01GM116254 to X.S.), the US Department of Health and Human Services contract HHSN272201400004C (NIAID Centers of Excellence for Influenza Research and Surveillance) and the Defense Advanced Research Projects Agency (Grant HR0011-10-00 to R.D.C.).
Author information
Authors and Affiliations
Contributions
X.S., D.F.S. and R.D.C. conceived the method; X.S., H.J., M.R.K. and Y.L. performed experiments; X.S., D.F.S. and R.D.C. analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15, Supplementary Tables 1–6 and Supplementary Note (PDF 13397 kb)
Rights and permissions
About this article
Cite this article
Song, X., Ju, H., Lasanajak, Y. et al. Oxidative release of natural glycans for functional glycomics. Nat Methods 13, 528–534 (2016). https://doi.org/10.1038/nmeth.3861
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3861
This article is cited by
-
Sialic acid O-acetylation patterns and glycosidic linkage type determination by ion mobility-mass spectrometry
Nature Communications (2023)
-
Pharmacokinetics of fucoidan and low molecular weight fucoidan from Saccharina japonica after oral administration to mice
Journal of Oceanology and Limnology (2023)
-
Chemoenzymatic modular assembly of O-GalNAc glycans for functional glycomics
Nature Communications (2021)
-
Glycan chip based on structure-switchable DNA linker for on-chip biosynthesis of cancer-associated complex glycans
Nature Communications (2021)
-
Comprehensive analysis of glycosphingolipid glycans by lectin microarrays and MALDI-TOF mass spectrometry
Nature Protocols (2021)