Abstract
Despite its importance for understanding human infertility and congenital diseases, early mammalian development has remained inaccessible to in toto imaging. We developed an inverted light-sheet microscope that enabled us to image mouse embryos from zygote to blastocyst, computationally track all cells and reconstruct a complete lineage tree of mouse pre-implantation development. We used this unique data set to show that the first cell fate specification occurs at the 16-cell stage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wu, Y. et al. Proc. Natl. Acad. Sci. USA 108, 17708–17713 (2011).
Krzic, U., Gunther, S., Saunders, T.E., Streichan, S.J. & Hufnagel, L. Nat. Methods 9, 730–733 (2012).
Keller, P.J., Schmidt, A.D., Wittbrodt, J. & Stelzer, E.H.K. Science 322, 1065–1069 (2008).
Piotrowska, K., Wianny, F., Pedersen, R.A. & Zernicka-Goetz, M. Development 128, 3739–3748 (2001).
Tabansky, I. et al. Curr. Biol. 23, 21–31 (2013).
Zernicka-Goetz, M. Nat. Rev. Mol. Cell Biol. 6, 919–928 (2005).
Plachta, N., Bollenbach, T., Pease, S., Fraser, S.E. & Pantazis, P. Nat. Cell Biol. 13, 117–123 (2011).
Motosugi, N., Bauer, T., Polanski, Z., Solter, D. & Hiiragi, T. Genes Dev. 19, 1081–1092 (2005).
Gardner, R.L. Development 128, 839–847 (2001).
Hiiragi, T. et al. Int. J. Dev. Biol. 50, 581–586, discussion 586–587 (2006).
Piotrowska-Nitsche, K., Perea-Gomez, A., Haraguchi, S. & Zernicka-Goetz, M. Development 132, 479–490 (2005).
Dietrich, J.-E. & Hiiragi, T. Development 134, 4219–4231 (2007).
Dietrich, J.E. & Hiiragi, T. Cells Tissues Organs 188, 46–51 (2008).
Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J. & Stelzer, E.H.K. Science 305, 1007–1009 (2004).
Weber, M. & Huisken, J. Curr. Opin. Genet. Dev. 21, 566–572 (2011).
Kaufmann, A., Mickoleit, M., Weber, M. & Huisken, J. Development 139, 3242–3247 (2012).
McGorty, R. et al. Opt. Express 23, 16142–16153 (2015).
Capoulade, J., Wachsmuth, M., Hufnagel, L. & Knop, M. Nat. Biotechnol. 29, 835–839 (2011).
Ichikawa, T. et al. PLoS ONE 8, e64506 (2013).
Udan, R.S., Piazza, V.G., Hsu, C.-W., Hadjantonakis, A.-K. & Dickinson, M.E. Development 141, 4406–4414 (2014).
Watanabe, T., Biggins, J.S., Tannan, N.B. & Srinivas, S. Development 141, 2279–2288 (2014).
Morris, S.A. et al. Proc. Natl. Acad. Sci. USA 107, 6364–6369 (2010).
Xenopoulos, P., Kang, M., Puliafito, A., Di Talia, S. & Hadjantonakis, A.-K. Cell Rep. 10, 1508–1520 (2015).
Dietrich, J.-E. et al. EMBO Rep. 16, 1005–1021 (2015).
Posfai, E., Tam, O.H. & Rossant, J. Curr. Top. Dev. Biol. 107, 1–37 (2014).
Abe, T. et al. Genesis 49, 579–590 (2011).
Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. Genesis 45, 593–605 (2007).
Wale, P.L. & Gardner, D.K. Reprod. Biomed. Online 21, 402–410 (2010).
Sommer, C., Straehle, C., Kothe, U. & Hamprecht, F.A. in Eighth IEEE International Symposium on Biomedical Imaging (ISBI) Proceedings 230–233 (2011).
Myronenko, A. & Song, X. IEEE Trans. Pattern Anal. Mach. Intell. 32, 2262–2275 (2010).
Rossant, J. & Tam, P.P.L. Dev. Cell 7, 155–164 (2004).
Rossant, J. & Tam, P.P.L. Development 136, 701–713 (2009).
McDole, K., Xiong, Y., Iglesias, P.A. & Zheng, Y. Dev. Biol. 355, 239–249 (2011).
Chen, B.-C. et al. Science 346, 1257998 (2014).
Booth, M.J. Light Sci. Appl. 3, e165 (2014).
Acknowledgements
We thank the mechanical and electronics workshop of the European Molecular Biology Laboratory (EMBL) for customized hardware, B. Klaus and S. Anders for help with statistical analysis, M. Eguren for cell fate assignment, E. Korotkevich for help with confocal imaging, B. Nijmeijer for mouse genotyping and EMBL's laboratory of animal resources for excellent support with mouse strains. The EMBL Advanced Light Microscopy Facility is acknowledged for support in image acquisition and analysis. This work was supported by funds from the German Research Foundation within in the priority program SPP 1384 “Mechanisms of genome haploidization” (DFG EL 246/4-1/-2) to J.E., by the European Union EU-FP7-MitoSys (Grant Agreement 241548) to J.E., and by the European Molecular Biology Laboratory (P.S., S.G., J.R., U.K., B.B., G.M., N.N., T.H., L.H. and J.E.). P.S., S.G. and N.N. were further supported by the EMBL Interdisciplinary Postdoc Programme (EIPOD) under Marie Curie Actions COFUND.
Author information
Authors and Affiliations
Contributions
J.E. supervised the work and originally conceived the project. L.H. and P.S. contributed to conception and design of the work. P.S. performed the experiments, designed and constructed the microscope and carried out the statistical analysis of the data as well as computer simulations. S.G. developed the software for cell tracking and applied it to the data. J.R. contributed to data acquisition and optimization of the microscope. T.H. supervised the embryological aspects of the work and carried out cell fate assignments. B.B., G.d.M. and N.N. developed and wrote the microscope software under the supervision of L.H. U.K. helped in the initial design of the microscope. J.E. and P.S. wrote the manuscript. All authors contributed to the design of the microscope or the experiments and interpretation of the data and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
P.S., U.K., L.H. and J.E. are co-inventors on a patent application that covers part of the microscopy technology described in this manuscript. L.H. and J.E. are scientific co-founders and advisors of an EMBL spin-off company, Luxendo GmbH, that makes light sheet–based microscopes commercially available.
Integrated supplementary information
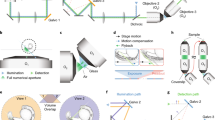
Supplementary Figure 1 Light path in the inverted light-sheet microscope.
Cross section through a model of the microscope’s sample mounting system: dash-dot lines indicate axis of excitation (Ex) and emission (Em) light paths, mirrors (M), illumination objective (IL), imaging objective (IM), flexible sealing ring (SR), immersion water reservoir (W), mounting base plate (BP).
Supplementary Figure 2 Environmental control incubator on the inverted light-sheet microscope.
Model of microscope’s sample mounting system with an incubator enabling precise temperature control as well as control of CO2 concentration in the atmosphere. Note the bottom plate of the incubator (shown in black) that minimizes heat transfer to the optical table. Openings (not shown but indicated by dashed line) on top and on the side of the incubator are fitted with sealed doors (not shown on the model).
Supplementary Figure 3 Light path of the inverted light-sheet microscope.
Schematic representation of inverted light-sheet microscope light path: lasers (L1, L2), acousto-optical modulator (AOM), clean-up filter (CF), glass plates (GP), kinematic mirrors (KM), dichroic mirrors (DM), optical fiber (F), filter wheel with neutral density filters (FW), polarizing beam splitters (PBS), fixed mirrors (M), beam expander (BE), mechanical shutters (SH), galvanometric scanning mirror (SM), scan lens (SL), tube lenses (TL), excitation light (Ex), illumination objective (IL), imaging objective (IM), motorized filter wheel with emission filters (MFW), emitted light (Em), field stop (FS), relay lenses (RL1, RL2), emission filters (EM1, EM2), sCMOS camera (sCMOS). Position of the EGFP and mCherry channels on the camera chip is indicated in inset.
Supplementary Figure 4 Point spread function of the inverted light-sheet microscope.
(a) Point spread function of the inverted light-sheet microscope system obtained by averaging images of fluorescent beads. (b) Intensity profile of the point spread function along the x- and y-axis.
Supplementary Figure 5 Live mice born after transfer of imaged embryos.
(a) Live-cell time-lapse imaging of mouse pre-implantation embryos expressing H2B-mCherry (red) and mG (green). Maximum intensity projections of first and last time-frames along the z-axis are shown. The cell surface marker was only imaged for the final division from 32 to ~64 cells. (b) Mice born after imaged embryos were implanted into pseudopregnant CD-1 females. Note that variable coat color is expected because imaged embryos are F2 hybrids from C3H and C57BL/6 strains.
Supplementary Figure 6 Confocal time-lapse imaging of pre-implantation embryos.
(a) Live-cell time-lapse confocal microscope imaging of mouse pre-implantation embryos expressing H2B-mCherry (red) and mG (not imaged). Maximum intensity projections of the first and the last time-frames along the z-axis and transmitted light images are shown. (b) Transmitted light pictures of imaged embryos and non-imaged control embryos kept under identical conditions. Note that because imaged embryos were arrested the time-lapse was stopped and mG transgene was not imaged.
Supplementary Figure 7 Lineage trees and fate assignment.
Lineage trees of mouse pre-implantation development from the one or two cell stage to the blastocyst for all tracked embryos (n = 12). Percentage of TE (red) or ICM (blue) cells derived from each cell is displayed on the tree by color code. All occurrences of cell death have been manually annotated and are indicated by terminated branches.
Supplementary Figure 8 Statistical analysis of lineage segregation.
Simplified representation of lineage trees. Color code represents how much a relative contribution of each branch to the ICM or TE lineages deviates from the whole embryo average (white, visible at one cell stage). Branches contributing only to ICM are shown in red and branches contributing only to TE are shown in blue. Probability that observed lineage segregation at 2-, 4-, 8-, 16- or 32-cell stage occurred by chance is shown on the right side of each tree. Probability was estimated using a permutation test where the observed tree was compared to 100,000 trees with randomized fates (Online Methods and Supplementary Note).
Supplementary Figure 9 Orientation of division axis correlates with cell fate.
(a) Live-cell time-lapse imaging of embryos expressing H2B-mCherry (white) and mG (not shown). Maximum intensity projections of selected time-points of the same embryo are shown. Examples of cells that contribute only to ICM (red), only TE (blue) or both (grey) lineages are shown together with their trajectories. Angle between the division axis and a line parallel to the embryo surface is illustrated by colored circle arcs. Cells dividing inwards have a positive and cells dividing outwards a negative directional division angle (Online Methods). Estimated embryo outline and center are shown by dashed circle and a cross. (b-d) Distribution of angles measured as illustrated in a. Cells were split at 16-cell stage into three categories depending on their contribution to cell lineages and analyzed separately. Cells that give rise only to ICM are shown in b, both lineages in c and only to TE in d. Random distribution is indicated by black line in each graph. Color code in graphs represents cells contributing to ICM (red), TE (blue) or both (grey) lineages at either 16 or 32-cell stage.
Supplementary Figure 10 Orientation of divisions at the transition from 8- to 16-cell stage is not random.
Distribution of division angles (Online Methods) during cell divisions from 2-cell stage up to 32-cell stage. Mitoses where cells divided parallel to the embryo surface have an angle of division 0°, whereas mitoses where cells divided in the direction of the inside/outside axis have a division angle of 90°. Random distribution is indicated by a black line. Probabilities that observed angles are coming from this random distribution are shown inside the graphs (Kolmogorov–Smirnov test, sample size inside graphs).
Supplementary Figure 11 Cells that contribute exclusively to TE or ICM lineage are preferentially coming from divisions along inside/outside axis.
Distribution of division angles (Online Methods) during cell divisions from 8- to 16- and 16- to 32-cells stage. Mitoses where cells divided parallel to the embryo surface have an angle of division 0°, whereas mitoses where cells divided in the direction of inside/outside axis have an angle of division 90°. Cells were split at 16-cell stage into three categories depending on their contribution to cell lineages and analyzed separately. Cells that give rise only to ICM are shown in the top two graphs, both lineages in the middle graphs or only to TE in the bottom two graphs. Random distribution is indicated by black line in each graph. Probabilities that observed angles are coming from this random distribution are shown inside the graphs (Kolmogorov–Smirnov test, sample size inside graphs).
Supplementary Figure 12 Cell fate can be predicted from directionality of cell divisions.
Schematic representation of simple rules that enable correct prediction of the fate of 84% of cells based solely on directional division angle (Online Methods). Progeny of all cells that divide at the 8- to 16-cell stage transition at an angle larger than 40° to the embryo surface will become ICM (top bar red, inside daughters) or TE (top bar blue, outside daughters). Fate of remaining cells (top bar grey) will be specified in next cell cycle where only inside daughters from divisions with an angle larger than 40° to the embryo surface will become ICM (bottom bar red). Remaining cells will become TE (bottom bar blue). Note that cells at 32-cell stage where one of the daughter cells dies or that gave rise to one TE and one ICM cell at 64-cell stage were not included in the analysis (7.8% of all cells).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12, Supplementary Results, Supplementary Discussion and Supplementary Note (PDF 2112 kb)
In toto live-cell time-lapse imaging of mouse pre-implantation development.
The movie displays maximum z- and x-projections of live-cell time-lapse imaging of mouse pre-implantation embryo expressing nuclear H2B-mCherry (red) and membrane mG (green) markers. Time stamps are relative to the metaphase to anaphase transition of the first mitosis at the two-cell stage (please note the additional polar body). Estimated embryonic development time is shown in brackets. (MP4 9600 kb)
Medium throughput In toto live-cell time-lapse imaging of mouse pre-implantation embryos.
The movie displays maximum z-projections of live-cell time-lapse imaging of multiple mouse pre-implantation embryos expressing nuclear H2B-mCherry (red) and membrane mG (green) markers. Our inverted light sheet microscope can acquire 20 embryos in parallel at a resolution used in this study. Time stamps are relative to the metaphase to anaphase transitions during the first mitosis at the 2-cell stage. Embryos were computationally synchronized with respect to time stamp zero. Estimated embryonic development time is shown in brackets. (MP4 12316 kb)
Computational reconstruction of mouse pre-implantation cell lineage.
The movie displays maximum projections of live-cell time-lapse imaging of mouse pre-implantation embryo expressing nuclear H2B-mCherry (white) and membrane mG (not shown) markers. The time stamps are relative to the metaphase to anaphase transition during the first mitosis at the 2-cell stage. Estimated embryonic development time is shown in brackets. Embryo was computationally registered and rotated such that ICM is facing to the right. Green spheres are drawn at the computationally determined nuclei centroids and their trajectories are illustrated by magenta lines (centroids of the polar body were computationally removed). (MP4 6816 kb)
Cell fate visualization during mouse pre-implantation development.
The movie displays maximum projections of live-cell time-lapse imaging of mouse pre-implantation embryo expressing nuclear H2B-mCherry (white) and membrane mG (not shown) markers. Time stamps are relative to the metaphase to anaphase transition during the first mitosis at the 2-cell stage. Estimated embryonic development time is shown in brackets. Embryo was computationally registered and rotated such that ICM is facing to the right. The colors of centroid spheres indicate the relative contribution of ICM (red) and TE (blue) lineages for each cell as determined by computationally back-tracking final cell fates at the end of the movie. White lines illustrate centroid trajectories of all nuclei (polar body trajectories were computationally removed). (MP4 6906 kb)
Cell fate visualization of selected cells during mouse pre-implantation development.
The movie displays maximum projections of live-cell time-lapse imaging of mouse pre-implantation embryo expressing nuclear H2B-mCherry (white) and membrane mG (not shown) markers. Time stamps are relative to the metaphase to anaphase transition during the first mitosis at the 2-cell stage. Estimated embryonic development time is shown in brackets. Embryo was computationally registered and rotated such that ICM is facing to the right. The colors of centroid spheres indicate the relative contribution of ICM (red) and TE (blue) lineages for selected cells as determined by computationally back-tracking final cell fates at the end of the movie. White lines illustrate centroid trajectories of selected nuclei. (MP4 3367 kb)
Supplementary Data
Nuclei coordinates, tracks and cell fates. (ZIP 9282 kb)
Supplementary Software
All source code (ZIP 67912 kb)
Rights and permissions
About this article
Cite this article
Strnad, P., Gunther, S., Reichmann, J. et al. Inverted light-sheet microscope for imaging mouse pre-implantation development. Nat Methods 13, 139–142 (2016). https://doi.org/10.1038/nmeth.3690
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3690
This article is cited by
-
High-resolution line-scan Brillouin microscopy for live imaging of mechanical properties during embryo development
Nature Methods (2023)
-
Light sheet fluorescence microscopy for the investigation of blood-sucking arthropods dyed via artificial membrane feeding
Parasites & Vectors (2022)
-
Multiscale light-sheet organoid imaging framework
Nature Communications (2022)
-
DaXi—high-resolution, large imaging volume and multi-view single-objective light-sheet microscopy
Nature Methods (2022)
-
A hybrid open-top light-sheet microscope for versatile multi-scale imaging of cleared tissues
Nature Methods (2022)