Abstract
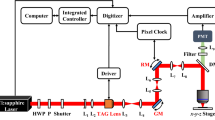
In vivo imaging at high spatiotemporal resolution is key to the understanding of complex biological systems. We integrated an optical phase-locked ultrasound lens into a two-photon fluorescence microscope and achieved microsecond-scale axial scanning, thus enabling volumetric imaging at tens of hertz. We applied this system to multicolor volumetric imaging of processes sensitive to motion artifacts, including calcium dynamics in behaving mouse brain and transient morphology changes and trafficking of immune cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Germain, R.N., Miller, M.J., Dustin, M.L. & Nussenzweig, M.C. Nat. Rev. Immunol. 6, 497–507 (2006).
Grewe, B.F. & Helmchen, F. Curr. Opin. Neurobiol. 19, 520–529 (2009).
Göbel, W., Kampa, B.M. & Helmchen, F. Nat. Methods 4, 73–79 (2007).
Grewe, B.F., Langer, D., Kasper, H., Kampa, B.M. & Helmchen, F. Nat. Methods 7, 399–405 (2010).
Cheng, A., Gonçalves, J.T., Golshani, P., Arisaka, K. & Portera-Cailliau, C. Nat. Methods 8, 139–142 (2011).
Ahrens, M.B., Orger, M.B., Robson, D.N., Li, J.M. & Keller, P.J. Nat. Methods 10, 413–420 (2013).
Schrödel, T., Prevedel, R., Aumayr, K., Zimmer, M. & Vaziri, A. Nat. Methods 10, 1013–1020 (2013).
Bouchard, M.B. et al. Nat. Photonics 9, 113–119 (2015).
Tang, J., Germain, R.N. & Cui, M. Proc. Natl. Acad. Sci. USA 109, 8434–8439 (2012).
Denk, W., Strickler, J.H. & Webb, W.W. Science 248, 73–76 (1990).
Botcherby, E.J. et al. Proc. Natl. Acad. Sci. USA 109, 2919–2924 (2012).
Katona, G. et al. Nat. Methods 9, 201–208 (2012).
Duemani Reddy, G., Kelleher, K., Fink, R. & Saggau, P. Nat. Neurosci. 11, 713–720 (2008).
McLeod, E., Hopkins, A.B. & Arnold, C.B. Opt. Lett. 31, 3155–3157 (2006).
Fan, Z. et al. Nat. Med. 16, 718–722 (2010).
Lämmermann, T. et al. Nature 498, 371–375 (2013).
Lindquist, R.L. et al. Nat. Immunol. 5, 1243–1250 (2004).
Zhu, J. et al. Immunity 37, 660–673 (2012).
Kastenmüller, W., Torabi-Parizi, P., Subramanian, N., Lämmermann, T. & Germain, R.N. Cell 150, 1235–1248 (2012).
Jung, S. et al. Mol. Cell. Biol. 20, 4106–4114 (2000).
Otsu, Y. et al. J. Neurosci. Methods 173, 259–270 (2008).
Chen, T.-W. et al. Nature 499, 295–300 (2013).
Nimmerjahn, A., Kirchhoff, F., Kerr, J.N. & Helmchen, F. Nat. Methods 1, 31–37 (2004).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Science 308, 1314–1318 (2005).
Guizar-Sicairos, M., Thurman, S.T. & Fienup, J.R. Opt. Lett. 33, 156–158 (2008).
Kim, J. et al. Nat. Methods 9, 96–102 (2012).
Adams, R. & Bischof, L. IEEE Trans. Pattern Anal. Mach. Intell. 16, 641–647 (1994).
Dombeck, D.A., Graziano, M.S. & Tank, D.W. J. Neurosci. 29, 13751–13760 (2009).
Acknowledgements
L.K. and M.C. thank W. Gan and H. Dana for many helpful discussions on cerebral cortex imaging, T.-W. Chen for help on data analysis in the visual cortex experiments, S. Peron and N.-L. Xu (Janelia Research Campus) for providing animals during system calibration, K. Ritola (Janelia Research Campus) for preparing GCaMP6 virus, K. Morris and J. Rouchard for virus injections, J. Liu for help on cluster computing, P. Keller for suggestions on data storage, B.-C. Chen and W. Legant for suggestions on data visualization, V. Iyer for support on ScanImage, D. Milkie for developing the custom Labview program, S. Sawtelle and L. Ramasamy for making the low-noise high-speed comparator, V. Goncharov for testing high-speed photodetectors, J. Freeman and F. Amat for help on data analysis and the Howard Hughes Medical Institute for funding the project. This work was supported in part by the Intramural Program of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health (NIH) (to R.N.G.), and NIH grant P41 E8015903-02S1 (to C.P.L.).
Author information
Authors and Affiliations
Contributions
M.C. devised the high-speed volumetric imaging method based on OPLUL and designed both the hardware and software. L.K. provided critical feedback to the system design. L.K. and M.C. built and calibrated the system. L.K. and J.P.L. designed the calcium imaging of the dendrites (V1 cortex). L.K. designed and performed calcium imaging of mouse cerebral cortexes. J.T., L.K., T.L. and R.N.G. designed the neutrophil experiments. C.P.L. designed the blood-flow imaging experiments. J.T. and R.N.G. designed the lymph node imaging experiments. J.T. and L.K. performed lymph node and neutrophil imaging. L.K. designed and performed microglia imaging experiments. L.K., Y.Y., J.T. and J.P.L. performed 3D data rendering and analysis. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18, Supplementary Table 1 and Supplementary Discussion (PDF 33384 kb)
2D cross-sectional in vivo calcium imaging of mouse V1 cortex (data used in Supplementary Fig. 8).
The frame was 250 × 130 μm2 (at depth 100-230 μm under the dura), and the frame rate was 893 Hz. The grating patterns used for visual stimulation are also shown. (MP4 20255 kb)
3D in vivo imaging of spontaneous neuronal network activity (dendrites and spines) at mouse V1 cortex.
The volume was 60 × 3.75 × 40 μm3 (at depth 107-147 μm under the dura) and the volume rate was 56 Hz. We displayed the maximum intensity projections of each volume in the video. (MOV 2070 kb)
Neuronal ensemble in Fig. 2a.
Maximal intensity projections of the ensemble in Supplementary Video 2 along the time axis. The volume was 60 × 3.75 × 40 μm3 at depth 107-147 μm under the dura. The video displays the x-y cross-sectional view of the volume. (AVI 209 kb)
3D in vivo calcium imaging of mouse visual cortex.
The volume was 375 × 375 × 130 μm3(at depth 100-230 μm under the dura) and the volume rate was 5.6 Hz. Maximum intensity projections of each volume are displayed along with the corresponding grating patterns used for the visual stimulation. (MOV 7585 kb)
Soma ensemble of V1 cortex in Supplementary Fig. 9.
The volume was 375 × 375 × 130 μm3 at depth 100-230 μm under the dura. Maximum intensity projections of the ensemble in Supplementary Video 4 along the time axis. Both the x-z cross-sectional view and the maximum intensity projections along x, y and z are shown. (MOV 463 kb)
3D in vivo imaging of the sensory-evoked neuronal network activity in the S1 cortex of awake mice under air-puff stimulations.
The volume was 375 × 112 × 130 μm3 (at depth 115-245 μm under the dura) and the volume rate was 14 Hz. Maximum intensity projections of each volume and the applied air-puff stimulation are shown. (MOV 1294 kb)
Soma ensemble (S1 cortex) in Fig. 2c.
Maximal intensity projections of the ensemble in Supplementary Video 6 along the time axis. The volume was 375 × 112 × 130 μm3 at depth 115-245 μm under the dura. The video shows both the x-z cross-sectional view and the maximum intensity projections. (MOV 230 kb)
3D in vivo imaging of the spontaneous dendritic network activity in the S1 cortex of awake mice.
The volume was 78 × 20 × 40 μm3 (at depth 40-80 μm under the dura) and the volume rate was 14 Hz. Maximum intensity projections of each volume are shown in the video. (MOV 1566 kb)
Dendrites/spines ensemble (S1 cortex) in Fig. 2e".
Maximal intensity projections of the ensemble in Supplementary Video 8 along the time axis. The volume was 78 × 20 × 40 μm3 at depth 40-80 μm under the dura. Both the x-y cross-sectional view and the maximum intensity projections are shown. (MOV 336 kb)
3D in vivo imaging of neuron ensemble activity in the M1 cortex of awake behaving mice.
The volume was 448 × 252 × 130 μm3 (at depth 150-280 μm under the dura) and the volume rate was 10 Hz. Maximum intensity projections of each volume are shown in the video. (MOV 8464 kb)
Soma ensemble (M1 cortex) in Fig. 2g.
Average intensity projections of the ensemble in Supplementary Video 10 along the time axis. The volume was 448 × 252 × 130 μm3 at depth 150-280 μm under the dura. The video displays the x-y cross sectional view of the volume. (MOV 104 kb)
2D cross-sectional in vivo imaging of neutrophil trafficking in the pial vein of mouse brain.
The imaging plane was 56 × 40 μm2 at depth 5-45 μm under the dura, at 90 degree with respect to the blood flow. The frame rate was 1 kHz. Green: GFP expressing neutrophil. The neutrophil image shown in Fig. 3a is from this video. (AVI 3001 kb)
3D in vivo imaging of neutrophils trafficking in the pial vein of mouse brain.
The volume was 112 × 38 × 40 μm3 (at depth 5-45 μm under the dura) and the volume rate was 39 Hz. Red: SR101 stained tissue, Green: GFP expressing neutrophils. The neutrophil images shown in Fig. 3b are from volume 5154-5159 of this video. The flow velocity of neutrophils in this video is 750-1243 μm/s. (MOV 5841 kb)
3D in vivo imaging of neutrophils trafficking and rolling in the pial vein of mouse brain.
The volume was 151 × 38 × 40 μm3 (at depth 5-45 μm under the dura) and the volume rate was 14 Hz. Red: SR101 stained tissue, Green: GFP expressing neutrophils. (MOV 4048 kb)
3D in vivo imaging of neutrophils trafficking in mouse brain cortex (S1).
The volume rate was 14 Hz. Red: SR101 stained astrocyte network, Green: GFP expressing neutrophils. We show the entire volume (151 × 38 × 40 μm3 at depth 50-90 μm under the dura) in the upper video and the sub-volume (z from 20 to 40 μm) in the lower video. The neutrophil images in Fig. 3c are from volume 93-95 and 97 of this video. (MOV 1690 kb)
Astrocyte network stained with SR101 in Supplementary Video 15.
The volume was 151 × 38 × 40 μm3 at depth 50-90 μm under the dura. The video displays the x-y cross-sectional view. (MOV 187 kb)
3D in vivo imaging of neutrophils trafficking in mouse ear vasculature.
The volume was 250 × 24 × 40 μm3 (at depth 65-105 μm under the surface) and the volume rate was 37 Hz. Red: blood plasma labeled with Q-dots (Qtracker® 655); Green: GFP expressing neutrophils. The neutrophil images shown in Supplementary Fig. 12 are from volume 7672-7679 of this video. (MOV 6505 kb)
3D in vivo imaging of neutrophils rolling along the mouse ear vasculature of a Lyz2gfp/+ B6.Albino transgenic mouse.
The volume was 250 × 47 × 40 μm3 (at depth 55-95 μm under the surface) and the volume rate was 19 Hz. Red: blood plasma labeled with Q-dots (Qtracker® 655); Green: GFP expressing neutrophils. (MOV 6679 kb)
3D in vivo imaging of neutrophils rolling along the mouse ear vasculature of a DsRed+/- Lyz2gfp/+ B6.Albino transgenic mouse.
The volume was 250 × 125 × 40 μm3 (at depth 50-90 μm under the surface) and the volume rate was 3.5 Hz. Red: dsRed expressing tissue stroma cells and blood vessel endothelium; Green: GFP expressing neutrophils. (MOV 2389 kb)
3D in vivo imaging of neutrophils in mouse ear vasculature and extravascular tissue.
The volume was 300 × 150 × 40 μm3 (at depth 50-90 μm under the surface) and the volume rate was 3.5 Hz. Red: blood plasma labeled with Q-dots (Qtracker® 655); Green: GFP expressing neutrophils; Blue: second harmonic generation (SHG) signals from the extracellular matrix. Data are shown in three colors on the left column, and in two colors on the right (no SHG) to highlight neutrophil dynamics. (MOV 1204 kb)
3D in vivo dendritic cell imaging inside mouse popliteal lymph node.
The volume was 500 × 250 × 40 μm3 (at depth 40-80 μm under the surface) and the volume rate was 1.7 Hz. (MOV 1250 kb)
3D in vivo dendritic cell imaging inside mouse popliteal lymph node (data used in Fig. 3d).
A bright cell appeared in volume 121. The volume was 125 × 125 × 40 μm3, at depth 55-95 μm under the surface. The volume rate was 1.7 Hz. The bright cell in volume 120, 121 and 149 of this video is also shown in Fig. 3d. (MOV 632 kb)
3D in vivo dendritic cell imaging inside mouse popliteal lymph node (data used in Supplementary Fig. 13).
The volume was 94 × 47 × 40 μm3, at depth 50-90 μm under the surface. The volume rate was 3.5 Hz. This cell (volume 499, 549, and 599 of this video) is also shown in Supplementary Fig. 13. (MOV 709 kb)
3D in vivo imaging of the lymphocytes (mostly memory T cells and natural killer cells) and intracellular fluorescent clusters in the popliteal lymph node of a Tbet:ZsGreen mouse.
The volume was 84 × 21 × 40 μm3 (at depth 100-140 μm under the surface) and the volume rate was 7 Hz. In this data set, the movement of two fluorescent clusters, which belong to different cells, is tracked with Imaris software and the time-color-coded traces show their trajectories. The moving speed of both clusters ranges from 0.1 to 6 μm /s, and the average speed is about 1 μm /s. (AVI 4445 kb)
3D in vivo imaging of resting microglia.
The volume was 150 × 150 × 40 μm3 (at depth 75-115 μm under the dura) and the volume rate was 1.7 Hz. We display the volume with the 3D voltex mode of Amira. The transient morphology of the two closely located microglial cells around the center (volume 51 of this video) is also shown in Supplementary Fig. 15. (AVI 14192 kb)
3D in vivo imaging of resting microglia surveying the microenvironment and monocytes patrolling around the cortex.
The volume was 150 × 150 × 40 μm3 (at depth 60-100 μm under the dura) and the volume rate was 1.7 Hz. Green: microglia and monocyte, Red: astrocytes stained with SR101. We display the volume with the 3D voltex mode of Amira. The transient morphology of the monocyte in volume 71-73 of this video is also shown in Supplementary Fig. 16. (AVI 5388 kb)
3D in vivo imaging of the microglia activated by the brain-blood-barrier disruption.
The volume was 150 × 75 × 40 μm3 (at depth 100-140 μm under the dura) and the volume rate was 3.5 Hz. Blue: microglia; Red: blood plasma labelled with Q-dots (Qtracker® 655). We show the volume with the 3D voltex mode of Amira. The bright red spot was from deposited Q-dots as a result of the brain blood barrier disruption. The microglia responded by directed subcellular structural changes. Data used in Fig. 3f was from the microglia to the left of the middle plane. The activated microglia in volume 51, 101 and 151 are shown in the left image of Fig. 3f, and those from volume 51, 56, 61 and 66 are shown in the right image of Fig. 3f. (AVI 12620 kb)
3D in vivo imaging of the microglia before being activated by neuron damage.
Resting microglia dynamics and spontaneous neuron activities. The volume was 187.5 × 187.5 × 40 μm3 (at depth 60-100 μm under the dura) and the volume rate was 1.7 Hz. The microglia and neurons are shown in orange and green, respectively. (AVI 14195 kb)
3D in vivo imaging of the microglia after being activated by neuron damage.
Transient structural changes of microglia after neuron damage. The volume was 187.5 × 187.5 × 40 μm3 (at depth 60-100 μm under the dura) and the volume rate was 1.7 Hz. Laser ablation was applied to the neuron that blinked frequently in the middle of the volume shown in Supplementary Video 28. (AVI 14191 kb)
Simultaneous imaging of the neuronal calcium signals and the change of blood vessel diameters in the S1 cortex of awake mice.
The volume was 210 × 70 × 40 μm3 (at depth 100-140 μm under the dura) and the volume rate was 14 Hz. Green: GCaMP6f-expressing neurons, Red: blood plasma labelled with Q-dots (Qtracker® 655). (MOV 2058 kb)
Neuron ensemble and vascular network of S1 cortex in Supplementary Fig. 17.
The volume was 210 × 70 × 40 μm3 at depth 100-140 μm under the dura. In this video, maximum intensity projections along the time axis were performed to reduce the 4D data to a 3D stack and the x-y cross-sectional view is displayed. (MOV 166 kb)
Supplementary Data
Data for Fig. a and Fig. b in Supplementary Discussion (XLSX 63 kb)
Supplementary Software
Matlab codes for image reconstruction (ZIP 145050 kb)
Source data
Rights and permissions
About this article
Cite this article
Kong, L., Tang, J., Little, J. et al. Continuous volumetric imaging via an optical phase-locked ultrasound lens. Nat Methods 12, 759–762 (2015). https://doi.org/10.1038/nmeth.3476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3476
This article is cited by
-
Dual-resonant scanning multiphoton microscope with ultrasound lens and resonant mirror for rapid volumetric imaging
Scientific Reports (2023)
-
Multifocal fluorescence video-rate imaging of centimetre-wide arbitrarily shaped brain surfaces at micrometric resolution
Nature Biomedical Engineering (2023)
-
Optical gearbox enabled versatile multiscale high-throughput multiphoton functional imaging
Nature Communications (2022)
-
Fast optical recording of neuronal activity by three-dimensional custom-access serial holography
Nature Methods (2022)
-
Imaging volumetric dynamics at high speed in mouse and zebrafish brain with confocal light field microscopy
Nature Biotechnology (2021)