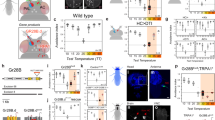
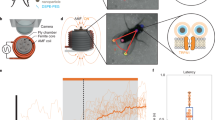
Abstract
Rapidly and selectively modulating the activity of defined neurons in unrestrained animals is a powerful approach in investigating the circuit mechanisms that shape behavior. In Drosophila melanogaster, temperature-sensitive silencers and activators are widely used to control the activities of genetically defined neuronal cell types. A limitation of these thermogenetic approaches, however, has been their poor temporal resolution. Here we introduce FlyMAD (the fly mind-altering device), which allows thermogenetic silencing or activation within seconds or even fractions of a second. Using computer vision, FlyMAD targets an infrared laser to freely walking flies. As a proof of principle, we demonstrated the rapid silencing and activation of neurons involved in locomotion, vision and courtship. The spatial resolution of the focused beam enabled preferential targeting of neurons in the brain or ventral nerve cord. Moreover, the high temporal resolution of FlyMAD allowed us to discover distinct timing relationships for two neuronal cell types previously linked to courtship song.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pfeiffer, B.D. et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 105, 9715–9720 (2008).
Jenett, A. et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2, 991–1001 (2012).
Lima, S.Q. & Miesenböck, G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121, 141–152 (2005).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Heisenberg, M. & Buchner, E. The role of retinula cell types in visual behavior of Drosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 117, 127–162 (1977).
Aravanis, A.M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Lin, J.Y., Knutsen, P.M., Muller, A., Kleinfeld, D. & Tsien, R.Y. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508 (2013).
Inagaki, H.K. et al. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 11, 325–332 (2014).
Klapoetke, N.C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Bernstein, J.G., Garrity, P.A. & Boyden, E.S. Optogenetics and thermogenetics: technologies for controlling the activity of targeted cells within intact neural circuits. Curr. Opin. Neurobiol. 22, 61–71 (2012).
Keene, A.C. & Masek, P. Optogenetic induction of aversive taste memory. Neuroscience 222, 173–180 (2012).
Marella, S., Mann, K. & Scott, K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron 73, 941–950 (2012).
Simon, J.C. & Dickinson, M.H. A new chamber for studying the behavior of Drosophila. PLoS ONE 5, e8793 (2010).
Straw, A.D. & Dickinson, M.H. Motmot, an open-source toolkit for realtime video acquisition and analysis. Source Code Biol. Med. 4, 5 (2009).
Pulver, S.R., Pashkovski, S.L., Hornstein, N.J., Garrity, P.A. & Griffith, L.C. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J. Neurophysiol. 101, 3075–3088 (2009).
Brand, A.H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Kitamoto, T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92 (2001).
Grigliatti, T.A., Hall, L., Rosenbluth, R. & Suzuki, D.T. Temperature-sensitive mutations in Drosophila melanogaster. Mol. Gen. Genet. 120, 107–114 (1973).
Poodry, C.A. & Edgar, L. Reversible alterations in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. J. Cell Biol. 81, 520–527 (1979).
Viswanath, V. et al. Opposite thermosensor in fruitfly and mouse. Nature 423, 822–823 (2003).
Hamada, F.N. et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008).
Mahr, A. & Aberle, H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns 6, 299–309 (2006).
Yamaguchi, S., Wolf, R., Desplan, C. & Heisenberg, M. Motion vision is independent of color in Drosophila. Proc. Natl. Acad. Sci. USA 105, 4910–4915 (2008).
Ellis, M.C., O'Neill, E.M. & Rubin, G.M. Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development 119, 855–865 (1993).
Bidaye, S.S., Machacek, C., Wu, Y. & Dickson, B.J. Neuronal control of Drosophila walking direction. Science 344, 97–101 (2014).
Ni, L. et al. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 500, 580–584 (2013).
Tang, X., Platt, M.D., Lagnese, C.M., Leslie, J.R. & Hamada, F.N. Temperature integration at the AC thermosensory neurons in Drosophila. J. Neurosci. 33, 894–901 (2013).
Gallio, M., Ofstad, T.A., Macpherson, L.J., Wang, J.W. & Zuker, C.S. The coding of temperature in the Drosophila brain. Cell 144, 614–624 (2011).
Clyne, J.D. & Miesenböck, G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 133, 354–363 (2008).
von Philipsborn, A.C. et al. Neuronal control of Drosophila courtship song. Neuron 69, 509–522 (2011).
Kohatsu, S., Koganezawa, M. & Yamamoto, D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69, 498–508 (2011).
Yu, J.Y., Kanai, M.I., Demir, E., Jefferis, G.S.X.E. & Dickson, B.J. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 20, 1602–1614 (2010).
Dankert, H., Wang, L., Hoopfer, E.D., Anderson, D.J. & Perona, P. Automated monitoring and analysis of social behavior in Drosophila. Nat. Methods 6, 297–303 (2009).
Branson, K., Robie, A., Bender, J., Perona, P. & Dickinson, M.H. High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451–457 (2009).
Friggi-Grelin, F. et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54, 618–627 (2003).
Straw, A.D., Branson, K., Neumann, T.R. & Dickinson, M.H. Multi-camera real-time three-dimensional tracking of multiple flying animals. J. R. Soc. Interface 8, 395–409 (2011).
Acknowledgements
We thank S. Bidaye for data on the VT50660-GAL4 genotype, the Institute of Molecular Pathology (IMP) workshop for help fabricating the hardware, M. Palfreyman and M. Dickinson for insightful discussion, L. Fenk for technological support and P. Masek for insight into IR activation of TrpA1. We thank J.H. Simpson (Howard Hughes Medical Institute, Janelia Farm Research Campus) for providing UAS-Shibirets1 flies. The fruit fly drawings in Figure 3 are modified from versions made available by Database Center for Life Science (DBCLS) under a CC 2.1 license. This work was supported by the Natural Sciences and Engineering Research Council of Canada by a postgraduate scholarship to D.E.B., European Research Council (ERC) Starting grant 281884 and Wiener Wissenschafts-, Forschungs- und Technologiefonds (WWTF) grant CS2011-029 to A.D.S., ERC Advanced grant 233306 to B.J.D. and IMP core funding.
Author information
Authors and Affiliations
Contributions
D.E.B., B.J.D. and A.D.S. conceived of the project. D.E.B., J.R.S., D.H., A.P. and A.D.S. developed the hardware and software. D.E.B. and D.H. performed experiments. All authors contributed to data analysis, interpretation and writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–16 and Supplementary Tables 1–5 (PDF 5575 kb)
FlyMAD: Rapid thermogenetic control of neuronal activity in freely-walking Drosophila
Summary of FlyMAD objectives, operation and results including thermogenetic silencing and activation. (MP4 10439 kb)
Silencing motoneurons with ShibireTS
Thermogenetic silencing of motoneurons reversibly disrupts locomotion. Genotype was +; OK371-Gal4/uas-ShibireTS1. (MP4 522 kb)
Silencing photoreceptors blocks the optomotor response
Thermogenetic silencing of visual neurons disrupts optomotor response. Genotype was Rh1-Gal4; UAS-ShibireTS. (MP4 4489 kb)
Activating Moonwalker neurons with TrpA1
Thermogenetic activation of moonwalker neurons induces backwards walking. Genotype was VT50660-Gal4; UAS-TrpA1. (MP4 864 kb)
Estimating the error of Through-The-Mirror (TTM) Tracking
In TTM tracking, the mirror movement command signal is proportional to the tracking error. The lower panel of this video shows the error in X and Y directions over time, while the upper panels show the wide and TTM camera views. (MP4 2641 kb)
Head-targeted heating induces proboscis extension from dopaminergic activation
Thermogenetic activation of flies with genotype TH-Gal4; UAS-TrpA1 causes proboscis extension. (MP4 19498 kb)
Activating song neurons in the VNC is stronger when targeting thorax than head
Thermogenetic activation of flies expressing TrpA1 in thoracic song neurons induces lower latency and more frequent singing when targeting the thorax than the head. (MP4 18944 kb)
P1-dependent courtship persists after stimulus ceases
A fly expressing TrpA1 in P1 performs courtship towards plasticine balls during and long after the thermogenetic stimulus is applied. Genotype is NP2361-Gal4; UAS>stop>TrpA1myc; fruFLP. (MP4 3585 kb)
pIP10-dependent courtship is closely linked to artificial activation
A fly expressing TrpA1 in pIP10 extends wings only when the thermogenetic stimulus is applied. Genotype is VT40347-Gal4; UAS>stop>TrpA1myc; fruFLP. (MP4 687 kb)
Supplementary Software
FlyMAD 0.9 installer. The file contains an installer for FlyMAD which depends on a computer system running a 64bit version of Ubuntu 12.04. The design files for the chamber, the optomotor drum, and the circuit board are included. (ZIP 2961 kb)
Rights and permissions
About this article
Cite this article
Bath, D., Stowers, J., Hörmann, D. et al. FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nat Methods 11, 756–762 (2014). https://doi.org/10.1038/nmeth.2973
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2973
This article is cited by
-
Quantum sensors for biomedical applications
Nature Reviews Physics (2023)
-
Multi-channel control of fruit fly behaviour
Nature Materials (2022)
-
Neural Control of Action Selection Among Innate Behaviors
Neuroscience Bulletin (2022)
-
Neural circuit mechanisms of sexual receptivity in Drosophila females
Nature (2021)
-
Sexual arousal gates visual processing during Drosophila courtship
Nature (2021)