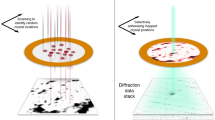
Abstract
X-ray free-electron laser (XFEL) sources enable the use of crystallography to solve three-dimensional macromolecular structures under native conditions and without radiation damage. Results to date, however, have been limited by the challenge of deriving accurate Bragg intensities from a heterogeneous population of microcrystals, while at the same time modeling the X-ray spectrum and detector geometry. Here we present a computational approach designed to extract meaningful high-resolution signals from fewer diffraction measurements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
03 June 2015
In the version of this article initially published, the authors claimed that with the tool cctbx.xfel, weak diffraction signals can be measured using fewer crystal specimens than are needed for the previously available program CrystFEL. However, there is not enough evidence to support this claim. The inaccurate statements have been corrected in the HTML and PDF versions of the article.
References
Neutze, R. et al. Nature 406, 752–757 (2000).
Alonso-Mori, R. et al. Proc. Natl. Acad. Sci. USA 109, 19103–19107 (2012).
Kern, J. et al. Science 340, 491–495 (2013).
Chapman, H.N. et al. Nature 470, 73–77 (2011).
Koopmann, R. et al. Nat. Methods 9, 259–262 (2012).
Redecke, L. et al. Science 339, 227–230 (2013).
Bourenkov, G.P. & Popov, A.N. Acta Crystallogr. D Biol. Crystallogr. 62, 58–64 (2006).
Sauter, N.K. et al. Acta Crystallogr. D Biol. Crystallogr. 69, 1274–1282 (2013).
White, T.A. et al. J. Appl. Cryst. 45, 335–341 (2012).
Boutet, S. et al. Science 337, 362–364 (2012).
Sauter, N.K., Grosse-Kunstleve, R.W. & Adams, P.D. J. Appl. Cryst. 37, 399–409 (2004).
Sauter, N.K. & Poon, B.K. J. Appl. Cryst. 43, 611–616 (2010).
Powell, H.R., Johnson, O. & Leslie, A.G. Acta Crystallogr. D Biol. Crystallogr. 69, 1195–1203 (2013).
Kirian, R.A. et al. Acta Crystallogr. A 67, 131–140 (2011).
Karplus, P.A. & Diederichs, K. Science 336, 1030–1033 (2012).
Kirian, R.A. et al. Opt. Express 18, 5713–5723 (2010).
Johansson, L.C. et al. Nat. Methods 9, 263–265 (2012).
Winkler, F.K., Schutt, C.E. & Harrison, S.C. Acta Crystallogr. A 35, 901–911 (1979).
Rossmann, M.G. et al. J. Appl. Cryst. 12, 570–581 (1979).
Zhu, D. et al. Appl. Phys. Lett. 101, 034103 (2012).
Inouye, K. J. Biochem. 112, 335–340 (1992).
Titani, K. et al. Nature 238, 35–37 (1972).
Boutet, S. & Williams, G.J. New J. Phys. 12, 035024 (2010).
Sierra, R.G. et al. Acta Crystallogr. D Biol. Crystallogr. 68, 1584–1587 (2012).
Bogan, M.J. Anal. Chem. 85, 3464–3471 (2013).
Hart, P. et al. Proc. SPIE 8504, 85040C (2012).
Maia, F.R.N.C. Nat. Methods 9, 854–855 (2012).
Zhang, Z. et al. J. Appl. Cryst. 39, 112–119 (2006).
Steller, I., Bolotovsky, R. & Rossmann, M.G. J. Appl. Cryst. 30, 1036–1040 (1997).
Rossmann, M.G. & van Beek, C.G. Acta Crystallogr. D Biol. Crystallogr. 55, 1631–1640 (1999).
Sauter, N.K., Grosse-Kunstleve, R.W. & Adams, P.D. J. Appl. Cryst. 39, 158–168 (2006).
Kern, J. et al. Proc. Natl. Acad. Sci. USA 109, 9721–9726 (2012).
Giordano, R. et al. Acta Crystallogr. D Biol. Crystallogr. 68, 649–658 (2012).
Diederichs, K. & Karplus, P.A. Acta Crystallogr. D Biol. Crystallogr. 69, 1215–1222 (2013).
Paithankar, K.S. et al. Acta Crystallogr. D Biol. Crystallogr. 67, 608–618 (2011).
White, T.A. et al. Acta Crystallogr. D Biol. Crystallogr. 69, 1231–1240 (2013).
Nave, C. Acta Crystallogr. D Biol. Crystallogr. 54, 848–853 (1998).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Emma, P. et al. Nat. Photonics 4, 641–647 (2010).
Greenhough, T.J. & Helliwell, J.R. J. Appl. Cryst. 15, 338–351 (1982).
Greenhough, T.J. & Helliwell, J.R. J. Appl. Cryst. 15, 493–508 (1982).
Greenhough, T.J., Helliwell, J.R. & Rule, S.A. J. Appl. Cryst. 16, 242–250 (1983).
Ren, Z. & Moffat, K. J. Appl. Cryst. 28, 461–481 (1995).
Dauter, Z. Acta Crystallogr. D Biol. Crystallogr. 55, 1703–1717 (1999).
Diederichs, K. Acta Crystallogr. D Biol. Crystallogr. 65, 535–542 (2009).
Schreurs, A.M.M., Xian, X. & Kroon-Batenburg, L.M.J. J. Appl. Cryst. 43, 70–82 (2009).
Porta, J. et al. Acta Crystallogr. D Biol. Crystallogr. 67, 628–638 (2011).
Bolotovsky, R. & Coppens, P. J. Appl. Cryst. 30, 65–70 (1997).
Leslie, A.G.W. Acta Crystallogr. D Biol. Crystallogr. 62, 48–57 (2006).
Kahn, R. et al. J. Appl. Cryst. 15, 330–337 (1982).
Brünger, A.T. Nature 355, 472–475 (1992).
Strüder, L. et al. Nucl. Instrum. Methods Phys. Res. A 614, 483–496 (2010).
Huang, T.C. et al. J. Appl. Cryst. 26, 180–184 (1993).
McCoy, A.J. et al. J. Appl. Cryst. 40, 658–674 (2007).
Adams, P.D. et al. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
English, A.C. et al. Proteins 37, 628–640 (1999).
Terwilliger, T.C. et al. Acta Crystallogr. D Biol. Crystallogr. 64, 61–69 (2008).
Afonine, P.V. et al. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Chen, V.B. et al. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Urzhumtseva, L. et al. Acta Crystallogr. D Biol. Crystallogr. 65, 297–300 (2009).
Afonine, P.V. et al. J. Appl. Cryst. 43, 669–676 (2010).
Acknowledgements
This work was supported by US National Institutes of Health (NIH) grants GM095887 and GM102520 and Director, Office of Science, US Department of Energy (DOE) under contract DE-AC02-05CH11231 for data-processing methods (N.K.S.); Director, DOE Office of Science, Office of Basic Energy Sciences (OBES), Chemical Sciences, Geosciences and Biosciences Division (CSGB) under contract DE-AC02-05CH11231 (J.Y. and V.K.Y.); NIH grant GM055302 (V.K.Y.); and NIH grant P41GM103393 (U.B.). Sample injection was supported by LCLS (M.J.B. and D.W.S.) and the Atomic, Molecular and Optical Science program, CSGB Division, OBES, DOE (M.J.B.), and through the SLAC National Accelerator Laboratory Directed Research and Development program (M.J.B. and H.L.). J.M. was supported by the Artificial Leaf Project Umeå (K&A Wallenberg Foundation), the Solar Fuels Strong Research Environment Umeå (Umeå University), Vetenskapsrådet and Swedish Energy Agency (Energimyndigheten). Experiments were carried out at the LCLS at SLAC, an Office of Science User Facility operated for the DOE by Stanford University. We thank A. Perazzo, M. Dubrovin, I. Ofte, and A. Salnikov for collaboration on data analysis, and C. Kenney for expertise related to the CSPAD detector.
Author information
Authors and Affiliations
Contributions
J. Hattne, J.K., J.Y., U.B., V.K.Y., P.D.A. and N.K.S. conceived of the new data-processing methods and analyzed the data; J. Hattne, N.E., R.J.G., A.S.B., R.W.G.-K., P.H.Z., M.M., P.D.A. and N.K.S. wrote the data-processing software; U.B., J.Y., V.K.Y., J.K., R.A.-M., J.M., A.Z., N.K.S., G.J.W., S.B., A.R.F., A.M., D.M., D.W.S., W.E.W. and M.J.B. designed the experiment; R.T., C.G., J. Hellmich, D.D., A.L., G.H., J.K. and A.Z. prepared samples; S.B., J.E.K., M.M., M.M.S., G.J.W. operated the CXI instrument; M.J.B., H.L., R.G.S., J.K., J.M., B.L.-K., S.G., R.T., C.G., J. Hellmich, J.S., D.W.S., A.M. and G.J.W. developed, tested and ran the sample delivery system; R.A.-M., U.B., M.J.B., S.B., N.E., R.J.G., P.G., C.G.,S.G., G.H., J.Hattne., J.Hellmich, J.K., J.E.K., H.L., A.L., B.L.-K., D.M., M.M., J.M., N.K.S., M.M.S., J.S., R.G.S., D.S., R.T., T.-C.W., G.J.W., V.K.Y., J.Y. and A.Z. performed the LCLS experiment; J. Hattne, N.E., J.K., J.Y., U.B., V.K.Y., P.D.A. and N.K.S. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2, Supplementary Tables 1–3, and Supplementary Note (PDF 447 kb)
Source data
Rights and permissions
About this article
Cite this article
Hattne, J., Echols, N., Tran, R. et al. Accurate macromolecular structures using minimal measurements from X-ray free-electron lasers. Nat Methods 11, 545–548 (2014). https://doi.org/10.1038/nmeth.2887
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2887
This article is cited by
-
Growing and making nano- and microcrystals
Nature Protocols (2023)
-
Chemical crystallography by serial femtosecond X-ray diffraction
Nature (2022)
-
Room temperature XFEL crystallography reveals asymmetry in the vicinity of the two phylloquinones in photosystem I
Scientific Reports (2021)
-
Structural characterization of a highly-potent V3-glycan broadly neutralizing antibody bound to natively-glycosylated HIV-1 envelope
Nature Communications (2018)
-
Towards in cellulo virus crystallography
Scientific Reports (2018)