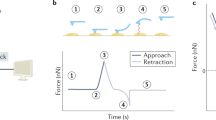
Abstract
A current challenge in the life sciences is to understand how biological systems change their structural, biophysical and chemical properties to adjust functionality. Addressing this issue has been severely hampered by the lack of methods capable of imaging biosystems at high resolution while simultaneously mapping their multiple properties. Recent developments in force-distance (FD) curve–based atomic force microscopy (AFM) now enable researchers to combine (sub)molecular imaging with quantitative mapping of physical, chemical and biological interactions. Here we discuss the principles and applications of advanced FD-based AFM tools for the quantitative multiparametric characterization of complex cellular and biomolecular systems under physiological conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ludwig, M., Dettmann, W. & Gaub, H.E. Atomic force microscope imaging contrast based on molecular recognition. Biophys. J. 72, 445–448 (1997). Pioneering work showing for the first time that FD-based AFM can be applied to image micropatterned surfaces that have been functionalized with receptors (streptavidin) and to map the specific interactions of the receptors with ligands (biotin) functionalizing the microscope tip.
Gad, M., Itoh, A. & Ikai, A. Mapping cell wall polysaccharides of living microbial cells using atomic force microscopy. Cell Biol. Int. 21, 697–706 (1997).
Heinz, W.F. & Hoh, J.H. Spatially resolved force spectroscopy of biological surfaces using the atomic force microscope. Trends Biotechnol. 17, 143–150 (1999).
Grandbois, M., Dettmann, W., Benoit, M. & Gaub, H.E. Affinity imaging of red blood cells using an atomic force microscope. J. Histochem. Cytochem. 48, 719–724 (2000).
Hinterdorfer, P. & Dufrêne, Y.F. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods 3, 347–355 (2006).
Roos, W.H., Bruinsma, R. & Wuite, G.J.L. Physical virology. Nat. Phys. 6, 733–743 (2010).
Gerber, C. & Lang, H.P. How the doors to the nanoworld were opened. Nat. Nanotechnol. 1, 3–5 (2006).
Müller, D.J. & Dufrêne, Y.F. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology. Nat. Nanotechnol. 3, 261–269 (2008).
Butt, H.-J., Cappella, B. & Kappl, M. Force measurements with the atomic force microscope: technique, interpretation and applications. Surf. Sci. Rep. 59, 1–152 (2005). An excellent and extensive review describing the potential possibilities and caveats of force measurements with the AFM. A must for all of those dealing with force measurements.
Müller, D.J., Helenius, J., Alsteens, D. & Dufrêne, Y.F. Force probing surfaces of living cells to molecular resolution. Nat. Chem. Biol. 5, 383–390 (2009).
Viani, M.B. et al. Small cantilevers for force spectroscopy of single molecules. J. Appl. Phys. 86, 2258–2262 (1999).
Viani, M.B. et al. Probing protein-protein interactions in real time. Nat. Struct. Biol. 7, 644–647 (2000).
Dong, M. & Sahin, O. A nanomechanical interface to rapid single-molecule interactions. Nat. Commun. 2, 247 (2011).
Martínez-Martín, D., Herruzo, E.T., Dietz, C., Gomez-Herrero, J. & Garcia, R. Noninvasive protein structural flexibility mapping by bimodal dynamic force microscopy. Phys. Rev. Lett. 106, 198101 (2011).
Martínez-Martín, D. et al. Resolving structure and mechanical properties at the nanoscale of viruses with frequency modulation atomic force microscopy. PLoS ONE 7, e30204 (2012).
Butt, H.-J. & Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 6, 1–7 (1995).
Florin, E.-L. et al. Sensing specific molecular interactions with the atomic force microscope. Biosens. Bioelectron. 10, 895–901 (1995).
Lee, G.U., Kidwell, D.A. & Colton, R.J. Sensing discrete streptavidin-biotin interactions with atomic force microscopy. Langmuir 10, 354–357 (1994).
Moy, V.T., Florin, E.-L. & Gaub, H.E. Intermolecular forces and energies between ligands and receptors. Science 266, 257–259 (1994).
Dammer, U. et al. Specific antigen/antibody interactions measured by force microscopy. Biophys. J. 70, 2437–2441 (1996).
Frisbie, C.D., Rozsnyai, L.F., Noy, A., Wrighton, M.S. & Lieber, C.M. Functional group imaging by chemical force microscopy. Science 265, 2071–2074 (1994).
Lee, H., Scherer, N.F. & Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 103, 12999–13003 (2006).
Dague, E. et al. Chemical force microscopy of single live cells. Nano Lett. 7, 3026–3030 (2007).
Dorobantu, L.S., Bhattacharjee, S., Foght, J.M. & Gray, M.R. Atomic force microscopy measurement of heterogeneity in bacterial surface hydrophobicity. Langmuir 24, 4944–4951 (2008).
Frederix, P.L. et al. Atomic force bio-analytics. Curr. Opin. Chem. Biol. 7, 641–647 (2003).
Tang, J. et al. Recognition imaging and highly ordered molecular templating of bacterial S-layer nanoarrays containing affinity-tags. Nano Lett. 8, 4312–4319 (2008).
Almqvist, N. et al. Elasticity and adhesion force mapping reveals real-time clustering of growth factor receptors and associated changes in local cellular rheological properties. Biophys. J. 86, 1753–1762 (2004).
Arnal, L. et al. Adhesin contribution to nanomechanical properties of the virulent Bordetella pertussis envelope. Langmuir 28, 7461–7469 (2012).
Friedsam, C., Del Campo Bécares, A., Jonas, U., Gaub, H.E. & Seitz, M. Polymer functionalized AFM tips for long-term measurements in single-molecule force spectroscopy. ChemPhysChem 5, 388–393 (2004).
Zimmermann, J.L., Nicolaus, T., Neuert, G. & Blank, K. Thiol-based, site-specific and covalent immobilization of biomolecules for single-molecule experiments. Nat. Protoc. 5, 975–985 (2010).
Bergkvist, M. & Cady, N.C. Chemical functionalization and bioconjugation strategies for atomic force microscope cantilevers. Methods Mol. Biol. 751, 381–400 (2011).
Barattin, R. & Voyer, N. Chemical modifications of atomic force microscopy tips. Methods Mol. Biol. 736, 457–483 (2011).
Bell, G.I. Models for the specific adhesion of cells to cells. Science 200, 618–627 (1978).
Evans, E. Probing the relation between force—lifetime—and chemistry in single molecular bonds. Annu. Rev. Biophys. Biomol. Struct. 30, 105–128 (2001). The energy landscape of a weak bond along a dissociation pathway is explored through Brownian-thermal excitations. This Review explains how dynamic force spectroscopy can be applied to probe the complex relation between force, lifetime and chemistry in single biomolecular bonds and to probe the bond's energy barriers that are difficult or impossible to detect in near-equilibrium assays.
Evans, E.A. & Calderwood, D.A. Forces and bond dynamics in cell adhesion. Science 316, 1148–1153 (2007).
Evans, E. Energy landscapes of biomolecular adhesion and receptor anchoring at interfaces explored with dynamic force spectroscopy. Faraday Discuss. 111, 1–16 (1999).
Dudko, O.K., Hummer, G. & Szabo, A. Intrinsic rates and activation free energies from single-molecule pulling experiments. Phys. Rev. Lett. 96, 108101 (2006).
Dudko, O.K., Hummer, G. & Szabo, A. Theory, analysis, and interpretation of single-molecule force spectroscopy experiments. Proc. Natl. Acad. Sci. USA 105, 15755–15760 (2008).
Medalsy, I., Hensen, U. & Müller, D.J. Imaging and quantifying chemical and physical properties of native proteins at molecular resolution by force–volume AFM. Angew. Chem. Int. Ed. Engl. 50, 12103–12108 (2011). This work applies FD-based AFM to image proteins in physiological relevant conditions and to simultaneously map their multiple chemical and physical properties at high resolution (∼1–2 nm). It is also shown that FD-based AFM is sufficiently sensitive to image single polypeptide loops of native proteins in their fully extended state and to mechanically adjust the conformation of single loops.
Hansma, P.K., Schitter, G., Fantner, G.E. & Prater, C. Applied physics. High-speed atomic force microscopy. Science 314, 601–602 (2006).
Ando, T. High-speed atomic force microscopy coming of age. Nanotechnology 23, 062001 (2012).
Dietz, C. et al. Nanotomography with enhanced resolution using bimodal atomic force microscopy. Appl. Phys. Lett. 92, 143107 (2008).
Dong, M., Husale, S. & Sahin, O. Determination of protein structural flexibility by microsecond force spectroscopy. Nat. Nanotechnol. 4, 514–517 (2009). Specially designed torsional harmonic cantilevers have been applied to perform high-speed force spectroscopic measurements while imaging purple membranes extracted from Halobacterium salinarum by AFM. Topographic and simultaneously recorded flexibility maps measure the Young's modulus of the two surfaces of the light-driven proton-pump bacteriorhodopsin that is embedded in the purple membrane.
Fukuma, T., Ueda, Y., Yoshioka, S. & Asakawa, H. Atomic-scale distribution of water molecules at the mica-water interface visualized by three-dimensional scanning force microscopy. Phys. Rev. Lett. 104, 016101 (2010).
Garcia, R. & Herruzo, E.T. The emergence of multifrequency force microscopy. Nat. Nanotechnol. 7, 217–226 (2012). An excellent overview of recent advances of multifrequency force microscopy. As discussed in our Review, these advances bring great potential to further improve the speed and sensitivity of FD-based AFM.
Jaafar, M. et al. Drive-amplitude-modulation atomic force microscopy: from vacuum to liquids. Beilstein J. Nanotechnol. 3, 336–344 (2012).
Giessibl, F.J. Forces and frequency shifts in atomic-resolution dynamic-force microscopy. Phys. Rev. B Condens. Matter Mater. Phys. 56, 16010–16015 (1997).
Thomas, W.E., Vogel, V. & Sokurenko, E. Biophysics of catch bonds. Annu. Rev. Biophys. 37, 399–416 (2008).
Kong, F. et al. Cyclic mechanical reinforcement of integrin-ligand interactions. Mol. Cell 49, 1060–1068 (2013).
Evans, E., Leung, A., Heinrich, V. & Zhu, C. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc. Natl. Acad. Sci. USA 101, 11281–11286 (2004).
Medalsy, I.D. & Müller, D.J. Nanomechanical properties of proteins and membranes depend on loading rate and electrostatic interactions. ACS Nano 7, 2642–2650 (2013).
Polyakov, P. et al. Automated force volume image processing for biological samples. PLoS ONE 6, e18887 (2011).
Roduit, C. et al. OpenFovea: open-source AFM data processing software. Nat. Methods 9, 774–775 (2012).
Weisenhorn, A.L., Khorsandi, M., Kasas, S., Gotzos, V. & Butt, H.-J. Deformation and height anomaly of soft surfaces studied with an AFM. Nanotechnology 4, 106–113 (1993).
Rotsch, C., Jacobson, K. & Radmacher, M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by using atomic force microscopy. Proc. Natl. Acad. Sci. USA 96, 921–926 (1999).
Végh, A.G. et al. Spatial and temporal dependence of the cerebral endothelial cells elasticity. J. Mol. Recognit. 24, 422–428 (2011).
Picas, L., Rico, F., Deforet, M. & Scheuring, S. Structural and mechanical heterogeneity of the erythrocyte membrane reveals hallmarks of membrane stability. ACS Nano 7, 1054–1063 (2013).
Heu, C., Berquand, A., Elie-Caille, C. & Nicod, L. Glyphosate-induced stiffening of HaCaT keratinocytes, a Peak Force Tapping study on living cells. J. Struct. Biol. 178, 1–7 (2012). One of the first times FD-based AFM was applied to characterize the structure and mechanical properties of human epidermal cells (HaCaT keratinocyte) under near-physiological conditions. The experiments show that herbicides induce cell membrane stiffening, the appearance of cytoskeleton structures at a subcellular level and membrane damage.
Matzke, R., Jacobson, K. & Radmacher, M. Direct, high-resolution measurement of furrow stiffening during division of adherent cells. Nat. Cell Biol. 3, 607–610 (2001).
Cross, S.E., Jin, Y.S., Rao, J. & Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2, 780–783 (2007).
Plodinec, M. et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 7, 757–765 (2012).
Francius, G. et al. Detection, localization, and conformational analysis of single polysaccharide molecules on live bacteria. ACS Nano 2, 1921–1929 (2008).
Touhami, A., Nysten, B. & Dufrêne, Y.F. Nanoscale mapping of the elasticity of microbial cells by atomic force microscopy. Langmuir 19, 4539–4543 (2003).
Pletikapic´, G., Berquand, A., Radic´, T.M. & Svetlic˘ic´, V. Quantitative nanomechanical mapping of marine diatom in seawater using peak force tapping atomic force microscopy. J. Phycol. 48, 174–185 (2012).
Roos, W.H. et al. Scaffold expulsion and genome packaging trigger stabilization of herpes simplex virus capsids. Proc. Natl. Acad. Sci. USA 106, 9673–9678 (2009). AFM images and FD curves were recorded to characterize the structure and mechanical stability of herpes simplex virus type 1 (HSV1) capsids. The measurements suggested that HSV1 capsids are stabilized after removal of the scaffold proteins and that this stabilization is triggered by the packaging of DNA but independent of the actual presence of DNA.
Carrasco, C. et al. Built-in mechanical stress in viral shells. Biophys. J. 100, 1100–1108 (2011).
Zink, M. & Grubmüller, H. Mechanical properties of the icosahedral shell of southern bean mosaic virus: a molecular dynamics study. Biophys. J. 96, 1350–1363 (2009).
Carrasco, C. et al. DNA-mediated anisotropic mechanical reinforcement of a virus. Proc. Natl. Acad. Sci. USA 103, 13706–13711 (2006).
Sullan, R.M., Li, J.K. & Zou, S. Direct correlation of structures and nanomechanical properties of multicomponent lipid bilayers. Langmuir 25, 7471–7477 (2009). FD-based AFM was applied to characterize the structure and mechanical responses of different phases and mixtures of lipid bilayers. The breakthrough forces, elastic moduli, adhesion forces and indentation of the microscope tip in dependence of the different phases in the lipid bilayers were systematically determined on the nanometer scale and presented as 2D maps.
An, H., Nussio, M.R., Huson, M.G., Voelcker, N.H. & Shapter, J.G. Material properties of lipid microdomains: force-volume imaging study of the effect of cholesterol on lipid microdomain rigidity. Biophys. J. 99, 834–844 (2010).
Möller, C., Allen, M., Elings, V., Engel, A. & Müller, D.J. Tapping-mode atomic force microscopy produces faithful high-resolution images of protein surfaces. Biophys. J. 77, 1150–1158 (1999).
Engel, A. & Müller, D.J. Observing single biomolecules at work with the atomic force microscope. Nat. Struct. Biol. 7, 715–718 (2000).
Rico, F., Su, C. & Scheuring, S. Mechanical mapping of single membrane proteins at submolecular resolution. Nano Lett. 11, 3983–3986 (2011).
Thoma, J., Bosshart, P., Pfreundschuh, M. & Müller, D.J. Out but not in: the large transmembrane β-barrel protein FhuA unfolds but cannot refold via β-hairpins. Structure 20, 2185–2190 (2012).
Sweers, K.K., van der Werf, K.O., Bennink, M.L. & Subramaniam, V. Atomic force microscopy under controlled conditions reveals structure of C-terminal region of a-synuclein in amyloid fibrils. ACS Nano 6, 5952–5960 (2012).
Zhang, S. et al. Coexistence of ribbon and helical fibrils originating from hIAPP20–29 revealed by quantitative nanomechanical atomic force microscopy. Proc. Natl. Acad. Sci. USA 110, 2798–2803 (2013).
Wegmann, S., Medalsy, I.D., Mandelkow, E. & Müller, D.J. The fuzzy coat of pathological human Tau fibrils is a two-layered polyelectrolyte brush. Proc. Natl. Acad. Sci. USA 110, E313–E321 (2013).
Wegmann, S. et al. Human Tau isoforms assemble into ribbon-like fibrils that display polymorphic structure and stability. J. Biol. Chem. 285, 27302–27313 (2010).
Alsteens, D. et al. High-resolution imaging of chemical and biological sites on living cells using peak force tapping atomic force microscopy. Langmuir 28, 16738–16744 (2012).
Alsteens, D. et al. Organization of the mycobacterial cell wall: a nanoscale view. Pflugers Arch. 456, 117–125 (2008).
Stroh, C. et al. Single-molecule recognition imaging microscopy. Proc. Natl. Acad. Sci. USA 101, 12503–12507 (2004).
Kim, H., Arakawa, H., Osada, T. & Ikai, A. Quantification of cell adhesion force with AFM: distribution of vitronectin receptors on a living MC3T3-E1 cell. Ultramicroscopy 97, 359–363 (2003).
Kim, H. et al. Quantification of the number of EP3 receptors on a living CHO cell surface by the AFM. Ultramicroscopy 106, 652–662 (2006).
Chtcheglova, L.A., Waschke, J., Wildling, L., Drenckhahn, D. & Hinterdorfer, P. Nano-scale dynamic recognition imaging on vascular endothelial cells. Biophys. J. 93, L11–L13 (2007).
Roduit, C. et al. Elastic membrane heterogeneity of living cells revealed by stiff nanoscale membrane domains. Biophys. J. 94, 1521–1532 (2008).
Dupres, V. et al. Nanoscale mapping and functional analysis of individual adhesins on living bacteria. Nat. Methods 2, 515–520 (2005).
Acknowledgements
This work was supported by the European Molecular Biology Organization (EMBO) (ALTF 506-2012); Swiss National Science Foundation (SNF); Belgian National Foundation for Scientific Research (FNRS); Université Catholique de Louvain; Belgian Federal Office for Scientific, Technical and Cultural Affairs (Interuniversity Poles of Attraction Programme); and Research Department of the Communauté Française de Belgique (Concerted Research Action).
Author information
Authors and Affiliations
Contributions
Manuscript and figures were assembled and outlined by Y.F.D. and D.J.M. All authors contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dufrêne, Y., Martínez-Martín, D., Medalsy, I. et al. Multiparametric imaging of biological systems by force-distance curve–based AFM. Nat Methods 10, 847–854 (2013). https://doi.org/10.1038/nmeth.2602
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2602
This article is cited by
-
Nanoscopic lignin mapping on cellulose nanofibers via scanning transmission electron microscopy and atomic force microscopy
Cellulose (2023)
-
Microbiologically Influenced Corrosion: A Concern for Oil and Gas Sector in Africa
Chemistry Africa (2023)
-
Electrochemical scanning probe microscopies for artificial photosynthesis
Nano Research (2023)
-
Effect of Probe Lifting Height in Jumping Mode AFM for Living Cell Imaging
Nanomanufacturing and Metrology (2023)
-
Atomic force microscopy-single-molecule force spectroscopy unveils GPCR cell surface architecture
Communications Biology (2022)