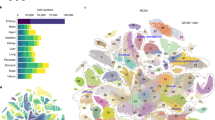
Abstract
The distinct cell types of multicellular organisms arise owing to constraints imposed by gene regulatory networks on the collective change of gene expression across the genome, creating self-stabilizing expression states, or attractors. We curated human expression data comprising 166 cell types and 2,602 transcription-regulating genes and developed a data-driven method for identifying putative determinants of cell fate built around the concept of expression reversal of gene pairs, such as those participating in toggle-switch circuits. This approach allows us to organize the cell types into their ontogenic lineage relationships. Our method identifies genes in regulatory circuits that control neuronal fate, pluripotency and blood cell differentiation, and it may be useful for prioritizing candidate factors for direct conversion of cell fate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Alberts, B. et al. Cells and genomes. in Molecular Biology of the Cell 3rd edn. Ch. 22 (Garland Science, New York, 1994).
Zhou, J.X. & Huang, S. Understanding gene circuits at cell-fate branch points for rational cell reprogramming. Trends Genet. 27, 55–62 (2011).
Kauffman, S.A. Control circuits for determination and transdetermination. Science 181, 310–318 (1973).
Kauffman, S.A., Shymko, R.M. & Trabert, K. Control of sequential compartment formation in Drosophila. Science 199, 259–270 (1978).
Zhang, P. et al. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc. Natl. Acad. Sci. USA 96, 8705–8710 (1999).
Huang, S. et al. Bifurcation dynamics in lineage-commitment in bipotent progenitor cells. Dev. Biol. 305, 695–713 (2007).
Geman, D., d'Avignon, C., Naiman, D.Q. & Winslow, R.L. Classifying gene expression profiles from pairwise mRNA comparisons. Stat. Appl. Genet. Mol. Biol. 3, Article 19 (2004).
Tan, A.C. et al. Simple decision rules for classifying human cancers from gene expression profiles. Bioinformatics 21, 3896–3904 (2005).
Price, N.D. et al. Highly accurate two-gene classifier for differentiating gastrointestinal stromal tumors and leiomyosarcomas. Proc. Natl. Acad. Sci. USA 104, 3414–3419 (2007).
Waddington, C.H. The Strategy of the Genes: A Discussion of Some Aspects of Theoretical Biology (Allen & Unwin, London, 1957).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
The ENCODE Project Consortium. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447, 799–816 (2007).
Chen, X. et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117 (2008).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Grass, J.A. et al. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. USA 100, 8811–8816 (2003).
Laslo, P. et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell 126, 755–766 (2006).
Hu, M. et al. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 11, 774–785 (1997).
Zhou, J.X., Brusch, L. & Huang, S. Predicting pancreas cell fate decisions and reprogramming with a hierarchical multi-attractor model. PLoS ONE 6, e14752 (2011).
Hosoya, T. et al. GATA-3 is required for early T lineage progenitor development. J. Exp. Med. 206, 2987–3000 (2009).
Miranda-Saavedra, D. & Göttgens, B. Transcriptional regulatory networks in haematopoiesis. Curr. Opin. Genet. Dev. 18, 530–535 (2008).
Swiers, G., Patient, R. & Loose, M. Genetic regulatory networks programming hematopoietic stem cells and erythroid lineage specification. Dev. Biol. 294, 525–540 (2006).
Feinberg, M.W. et al. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 26, 4138–4148 (2007).
Hoang, T. et al. Opposing effects of the basic helix-loop-helix transcription factor SCL on erythroid and monocytic differentiation. Blood 87, 102–111 (1996).
Ma, C. & Staudt, L.M. LAF-4 encodes a lymphoid nuclear protein with transactivation potential that is homologous to AF-4, the gene fused to MLL in t(4;11) leukemias. Blood 87, 734–745 (1996).
Nagasawa, M., Schmidlin, H., Hazekamp, M.G., Schotte, R. & Blom, B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur. J. Immunol. 38, 2389–2400 (2008).
Hagman, J., Belanger, C., Travis, A., Turck, C.W. & Grosschedl, R. Cloning and functional characterization of early B-cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev. 7, 760–773 (1993).
Zandi, S. et al. EBF1 is essential for B-lineage priming and establishment of a transcription factor network in common lymphoid progenitors. J. Immunol. 181, 3364–3372 (2008).
Lukin, K. et al. A dose-dependent role for EBF1 in repressing non-B-cell-specific genes. Eur. J. Immunol. 41, 1787–1793 (2011).
Dontje, W. et al. Delta-like1-induced Notch1 signaling regulates the human plasmacytoid dendritic cell versus T-cell lineage decision through control of GATA-3 and Spi-B. Blood 107, 2446–2452 (2006).
Rosa, A. et al. The interplay between the master transcription factor PU.1 and miR-424 regulates human monocyte/macrophage differentiation. Proc. Natl. Acad. Sci. USA 104, 19849–19854 (2007).
Wei, G. et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 35, 299–311 (2011).
Treiber, T. et al. Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription- independent poising of chromatin. Immunity 32, 714–725 (2010).
Duarte, N.C. et al. Global reconstruction of the human metabolic network based on genomic and bibliomic data. Proc. Natl. Acad. Sci. USA 104, 1777–1782 (2007).
Pardo, M. et al. An expanded Oct4 interaction network: implications for stem cell biology, development, and disease. Cell Stem Cell 6, 382–395 (2010).
Kashyap, V. et al. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 18, 1093–1108 (2009).
Li, J.-Y. et al. Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol. Cell Biol. 27, 8748–8759 (2007).
Sinkkonen, L. et al. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nat. Struct. Mol. Biol. 15, 259–267 (2008).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Ito, S. et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133 (2010).
Neph, S. et al. Circuitry and dynamics of human transcription factor regulatory networks. Cell 150, 1274–1286 (2012).
Wu, Z. & Irizarry, R.A. Stochastic models inspired by hybridization theory for short oligonucleotide arrays. J. Comput. Biol. 12, 882–893 (2005).
Nishikawa, S.I. et al. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125, 1747–1757 (1998).
Allen, C.D.C., Okada, T. & Cyster, J.G. Germinal-center organization and cellular dynamics. Immunity 27, 190–202 (2007).
Burkard, R., DellAmico, M. & Martello, S. Assignment Problems (SIAM, Philadelphia, 2009).
McLean, C.Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Acknowledgements
We thank R. Bressler (Institute for Systems Biology) for providing the interactive landscape visualization for the web page, T. Sauter and T. Schilling (University of Luxembourg) for the use of their computational resource, D. Galas and C. Carlberg for useful discussions and suggestions and E. Friederich and N. Vlassis for reading the manuscript; and we gratefully acknowledge these sources of funding: the Academy of Finland, project no. 132877 (M.N.), the University of Luxembourg, Tekes FiDiPro Program (S.A.K.), Alberta Innovates the Future (S.H.) and US National Institutes of Health–National Institute of General Medical Sciences grants R01GM072855 and P50GMO76547 (I.S.).
Author information
Authors and Affiliations
Contributions
M.H., M.N., R. Kramer and I.S. designed the gene-pair analysis, and M.H. and R. Kramer performed the analysis. M.H. and A.W.-B. designed the gene curation pipeline, and M.H., A.W.-B. and L.S. curated the genes. M.N., M.H., J.X.Z., S.A.K., S.H. and I.S. designed the clustering experiments and visualization of cell type dissimilarities. M.N. designed the branch-point placement algorithm. M.H. and M.N. compiled the ChIP-seq validations. M.H. and S.H. designed the reversal participation analysis. R. Kreisberg, M.H., M.N. and I.S. designed the content of the online resource. M.H., M.N., R. Kramer, S.H. and I.S. wrote the manuscript. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13, Supplementary Tables 4 and 6 and Supplementary Results (PDF 2503 kb)
Supplementary Table 1
Cell type and tissue ontology terms (XLS 50 kb)
Supplementary Table 2
Microarray samples mapped to ontology terms (XLS 247 kb)
Supplementary Table 3
The order of cell types as it appears in the heat maps presented (XLS 29 kb)
Supplementary Table 5
Functional evidence for a role in transcription regulation found in the gene-set curation (XLS 306 kb)
Supplementary Table 7
Identification of candidate toggle pairs (XLS 69 kb)
Supplementary Table 8
Rank-based differential expression analysis comparison using RCoS (XLS 710 kb)
Supplementary Table 9
Rank-based differential expression analysis comparison using RDAM (XLS 245 kb)
Supplementary Table 10
Public ChIP-seq data sets used (XLS 23 kb)
Supplementary Table 11
Genomic region enrichment results for GATA1 ChIP-seq data (XLS 1413 kb)
Supplementary Table 12
Genomic region enrichment results for TAL1 ChIP-seq data (XLS 713 kb)
Supplementary Table 13
Genomic region enrichment results for SPI1 ChIP-seq data (XLS 1936 kb)
Supplementary Table 14
Genomic region enrichment results for EBF1 ChIP-seq data (XLS 969 kb)
Supplementary Table 15
Genomic region enrichment results for GATA3 ChIP-seq data (XLS 3041 kb)
Supplementary Table 16
Mouse knockout phenotypes of Gata1, Tal1, Sfpi1, Ebf1 and Gata3 (XLS 114 kb)
Supplementary Table 17
Additional microarray data used for validation. (XLS 97 kb)
Supplementary Software
Online data resource and tool TREL. The online data resource and interactive tool (http://trel.systemsbiology.net/) encompassing pairwise comparisons of the genes and cell types presented in this article is available to explore transcriptome diversity in metazoa; this resource accompanied by a user guide and video tutorial. (ZIP 4901 kb)
Rights and permissions
About this article
Cite this article
Heinäniemi, M., Nykter, M., Kramer, R. et al. Gene-pair expression signatures reveal lineage control. Nat Methods 10, 577–583 (2013). https://doi.org/10.1038/nmeth.2445
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2445
This article is cited by
-
Immune-related lincRNA pairs predict prognosis and therapeutic response in hepatocellular carcinoma
Scientific Reports (2022)
-
Reference Module-Based Analysis of Ovarian Cancer Transcriptome Identifies Important Modules and Potential Drugs
Biochemical Genetics (2022)
-
Prediction of prognosis and immunotherapy response with a robust immune-related lncRNA pair signature in lung adenocarcinoma
Cancer Immunology, Immunotherapy (2022)
-
A signature of 24 aging‑related gene pairs predict overall survival in gastric cancer
BioMedical Engineering OnLine (2021)
-
Long non-coding RNA pairs to assist in diagnosing sepsis
BMC Genomics (2021)