Abstract
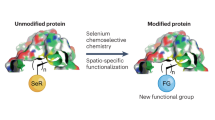
Selenium is essential to human life and occurs in selenoproteins as selenocysteine (Sec), the 21st amino acid. The selenium atom endows selenocysteine with unique biochemical properties, including a low pKa and a high reactivity with many electrophilic agents. Here we describe the introduction of selenocysteine into recombinant non-selenoproteins produced in Escherichia coli, as part of a small tetrapeptide motif at the C terminus. This selenocysteine-containing motif could subsequently be used as a protein tag for purification of the recombinant protein, selenolate-targeted labeling with fluorescent compounds or radiolabeling with either γ-emitting 75Se or short-lived positron emitters such as 11C. The results presented here thus show how a wide range of biotechnological applications can be developed starting from the insertion of selenocysteine into proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kryukov, G.V. et al. Characterization of mammalian selenoproteomes. Science 300, 1439–1443 (2003).
Stadtman, T.C. Selenocysteine. Annu. Rev. Biochem. 65, 83–100 (1996).
Böck, A. et al. Selenocysteine: the 21st amino acid. Mol. Microbiol. 5, 515–520 (1991).
Gieselman, M.D., Zhu, Y., Zhou, H., Galonic, D. & van der Donk, W.A. Selenocysteine derivatives for chemoselective ligations. Chembiochem 3, 709–716 (2002).
Hondal, R.J. & Raines, R.T. Semisynthesis of proteins containing selenocysteine. Methods Enzymol. 347, 70–83 (2002).
Lian, G. et al. Preparation and properties of a selenium-containing catalytic antibody as type I deiodinase mimic. J. Biol. Chem. 276, 28037–28041 (2001).
Müller, S. et al. The formation of diselenide bridges in proteins by incorporation of selenocysteine residues: biosynthesis and characterization of (Se)2-thioredoxin. Biochemistry 33, 3404–3412 (1994).
Arnér, E.S.J., Sarioglu, H., Lottspeich, F., Holmgren, A. & Böck, A. High-level expression in Escherichia coli of selenocysteine-containing rat thioredoxin reductase utilizing gene fusions with engineered bacterial-type SECIS elements and co-expression with the selA, selB and selC genes. J. Mol. Biol. 292, 1003–1016 (1999).
Gladyshev, V.N., Jeang, K-T. & Stadtman, T.C. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc. Natl Acad. Sci. USA 93, 6146–6151 (1996).
Zhong, L., Arnér, E.S.J., Ljung, J., Åslund, F. & Holmgren, A. Rat and calf thioredoxin reductase are homologous to glutathione reductase with a carboxyl-terminal elongation containing a conserved catalytically active penultimate selenocysteine residue. J. Biol. Chem. 273, 8581–8591 (1998).
Zhong, L., Arnér, E.S.J. & Holmgren, A. Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc. Natl. Acad. Sci. USA 97, 5854–5859 (2000).
Nordberg, J., Zhong, L., Holmgren, A. & Arnér, E.S.J. Mammalian thioredoxin reductase is irreversibly inhibited by dinitrohalobenzenes by alkylation of both the redox active selenocysteine and its neighboring cysteine residue. J. Biol. Chem. 273, 10835–10842 (1998).
Mutt, V. Vasoactive intestinal polypeptide and related peptides. Isolation and chemistry. Ann. NY Acad. Sci. 527, 1–19 (1988).
Simoncsits, A. et al. Synthesis, cloning and expression in Escherichia coli of artificial genes coding for biologically active elongated precursors of the vasoactive intestinal polypeptide. Eur. J. Biochem. 178, 343–350 (1988).
Heymann, P.W., Chapman, M.D., Aalberse, R.C., Fox, J.W. & Platts-Mills, T.A. Antigenic and structural analysis of group II allergens (Der f II and Der p II) from house dust mites (Dermatophagoides spp). J. Allergy Clin. Immunol. 83, 1055–1067 (1989).
Kalef, E., Walfish, P.G. & Gitler, C. Arsenical-based affinity chromatography of vicinal dithiol-containing proteins: purification of L1210 leukemia cytoplasmic proteins and the recombinant rat c-erb Aβ1 T3 receptor. Anal. Biochem. 212, 325–334 (1993).
Zhou, G.Y., Jauhiainen, M., Stevenson, K. & Dolphin, P.J. Human plasma lecithin:cholesterol acyltransferase. Preparation and use of immobilized p-aminophenylarsenoxide as a catalytic site-directed covalent ligand in enzyme purification. J. Chromatogr. 568, 69–83 (1991).
Hoffman, R.D. & Lane, M.D. Iodophenylarsine oxide and arsenical affinity chromatography: new probes for dithiol proteins. Application to tubulins and to components of the insulin receptor-glucose transporter signal transduction pathway. J. Biol. Chem. 267, 14005–14011 (1992).
Müller, S., Heider, J. & Böck, A. The path of unspecific incorporation of selenium in Escherichia coli. Arch. Microbiol. 168, 421–427 (1997).
Gespach, C., Bawab, W., de Cremoux, P. & Calvo, F. Pharmacology, molecular identification and functional characteristics of vasoactive intestinal peptide receptors in human breast cancer cells. Cancer Res. 48, 5079–5083 (1988).
Gromer, S., Arscott, L.D., Williams, C.H., Jr, Schirmer, R.H. & Becker, K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 273, 20096–20101 (1998).
Terpe, K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 60, 523–533 (2003).
Griffin, B.A., Adams, S.R. & Tsien, R.Y. Specific covalent labeling of recombinant protein molecules inside live cells. Science 281, 269–272 (1998).
Adams, S.R. et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 124, 6063–6076 (2002).
Arnér, E.S.J. Recombinant expression of mammalian selenocysteine-containing thioredoxin reductase and other selenoproteins in Escherichia coli. Methods Enzymol. 347, 226–235 (2002).
Ma, S., Caprioli, R.M., Hill, K.E. & Burk, R.F. Loss of selenium from selenoproteins: conversion of selenocysteine to dehydroalanine in vitro. J. Am. Soc. Mass Spectrom. 14, 593–600 (2003).
Fichna, J. & Janecka, A. Synthesis of target-specific radiolabeled peptides for diagnostic imaging. Bioconjug. Chem. 14, 3–17 (2003).
Okarvi, S.M. Recent progress in fluorine-18 labelled peptide radiopharmaceuticals. Eur. J. Nucl. Med. 28, 929–938 (2001).
Waibel, R. et al. Stable one-step technetium-99m labeling of His-tagged recombinant proteins with a novel Tc(I)-carbonyl complex. Nat. Biotechnol. 17, 897–901 (1999).
Kapanidis, A.N., Ebright, Y.W. & Ebright, R.H. Site-specific incorporation of fluorescent probes into protein: hexahistidine-tag-mediated fluorescent labeling with (Ni2+:nitrilotriacetic acid (n)-fluorochrome conjugates. J. Am. Chem. Soc. 123, 12123–12125 (2001).
Chua, K.Y. et al. Isolation of cDNA coding for the major mite allergen Der p II by IgE plaque immunoassay. Int. Arch. Allergy Appl. Immunol. 91, 118–123 (1990).
Arnér, E.S.J., Zhong, L. & Holmgren, A. Preparation and assay of mammalian thioredoxin and thioredoxin reductase. Methods Enzymol. 300, 226–239 (1999).
Said, S.I. & Mutt, V. Isolation from porcine intestinal wall of a vasoactive octacosapeptide related to secretin and to glucagon. Eur. J. Biochem. 28, 199–204 (1972).
Acknowledgements
We thank C. Gitler and E. Kalef, Weizmann Institute of Science, Rehovot, Israel, for helpful advice regarding PAO-Sepharose synthesis; M. Engberg for initial assistance in cloning work; E. Refai, Department of Medical Biochemistry and Biophysics, Karolinska Institute, for assistance and providing native VIP; and H. Grönlund, Department of Medicine, Clinical Immunology and Allergy, Karolinska Institute, for providing the Der p 2-6 His clone. This work was supported by the Karolinska Institute, the Swedish Cancer Society (projects 4056 and 4722), the Swedish Research Council for Medicine (projects 14527 and 14528), the Swedish Asthma and Allergy Associations Research Foundation, Åke Wibergs Foundation, Lars Hiertas Foundation, Konsul Th. C. Bergs Foundation, Magnus Bergvalls Foundation, Hesselmans Foundation and the Swedish Cancer and Asthma fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Johansson, L., Chen, C., Thorell, JO. et al. Exploiting the 21st amino acid—purifying and labeling proteins by selenolate targeting. Nat Methods 1, 61–66 (2004). https://doi.org/10.1038/nmeth707
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth707
This article is cited by
-
Preclinical PET imaging of EGFR levels: pairing a targeting with a non-targeting Sel-tagged Affibody-based tracer to estimate the specific uptake
EJNMMI Research (2016)
-
Tagging recombinant proteins with a Sel-tag for purification, labeling with electrophilic compounds or radiolabeling with 11C
Nature Protocols (2006)