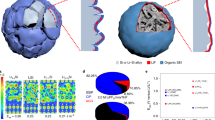
Abstract
Si-based Li-ion battery anodes have recently received great attention, as they offer specific capacity an order of magnitude beyond that of conventional graphite. The applications of this transformative technology require synthesis routes capable of producing safe and easy-to-handle anode particles with low volume changes and stable performance during battery operation. Herein, we report a large-scale hierarchical bottom-up assembly route for the formation of Si on the nanoscale—containing rigid and robust spheres with irregular channels for rapid access of Li ions into the particle bulk. Large Si volume changes on Li insertion and extraction are accommodated by the particle’s internal porosity. Reversible capacities over five times higher than that of the state-of-the-art anodes (1,950 mA h g−1) and stable performance are attained. The synthesis process is simple, low-cost, safe and broadly applicable, providing new avenues for the rational engineering of electrode materials with enhanced conductivity and power.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
19 March 2010
In the version of this Article originally published, the surname of A. Magasinski was spelled incorrectly. This has been corrected in the HTML and PDF versions of this Article.
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nature Mater. 7, 845–854 (2008).
Kang, B. & Ceder, G. Battery materials for ultrafast charging and discharging. Nature 458, 190–193 (2009).
Arico, A. S., Bruce, P., Scrosati, B., Tarascon, J. M. & Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater. 4, 366–377 (2005).
Chan, C. K. et al. High-performance lithium battery anodes using silicon nanowires. Nature Nanotech. 3, 31–35 (2008).
Poizot, P., Laruelle, S., Grugeon, S., Dupont, L. & Tarascon, J. M. Nano-sized transition-metaloxides as negative-electrode materials for lithium-ion batteries. Nature 407, 496–499 (2000).
Taberna, L., Mitra, S., Poizot, P., Simon, P. & Tarascon, J. M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nature Mater. 5, 567–573 (2006).
Oumellal, Y., Rougier, A., Nazri, G. A., Tarascon, J. M. & Aymard, L. Metal hydrides for lithium-ion batteries. Nature Mater. 7, 916–921 (2008).
Poizot, P., Laruelle, S., Grugeon, S., Dupont, L. & Tarascon, J. M. Searching for new anode materials for the Li-ion technology: Time to deviate from the usual path. J. Power Sources 97–8, 235–239 (2001).
Huggins, R. A. Lithium alloy negative electrodes. J. Power Sources 81, 13–19 (1999).
Liu, W. R., Yang, M. H., Wu, H. C., Chiao, S. M. & Wu, N. L. Enhanced cycle life of Si anode for Li-ion batteries by using modified elastomeric binder. Electrochem. Solid State Lett. 8, A100–A103 (2005).
Li, J., Lewis, R. B. & Dahn, J. R. Sodium carboxymethyl cellulose—a potential binder for Si negative electrodes for Li-ion batteries. Electrochem. Solid State Lett. 10, A17–A20 (2007).
Beattie, S. D., Larcher, D., Morcrette, M., Simon, B. & Tarascon, J. M. Si electrodes for Li-ion batteries—a new way to look at an old problem. J. Electrochem. Soc. 155, A158–A163 (2008).
Mazouzi, D., Lestriez, B., Roue, L. & Guyomard, D. Silicon composite electrode with high capacity and long cycle life. Electrochem. Solid State Lett. 12, A215–A218 (2009).
Obrovac, M. N. & Krause, L. J. Reversible cycling of crystalline silicon powder. J. Electrochem. Soc. 154, A103–A108 (2007).
Cui, L. F., Ruffo, R., Chan, C. K., Peng, H. L. & Cui, Y. Crystalline-amorphous core–shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 9, 491–495 (2009).
Kim, H. & Cho, J. Superior lithium electroactive mesoporous Si–carbon core–shell nanowires for lithium battery anode material. Nano Lett. 8, 3688–3691 (2008).
Oberdorster, G., Stone, V. & Donaldson, K. Toxicology of nanoparticles: A historical perspective. Nano Toxicol. 1, 2–25 (2007).
Stern, S. T. & McNeil, S. E. Nanotechnology safety concerns revisited. Toxicol. Sci. 101, 4–21 (2008).
Xing, W. B., Wilson, A. M., Eguchi, K., Zank, G. & Dahn, J. R. Pyrolyzed polysiloxanes for use as anode materials in lithium-ion batteries. J. Electrochem. Soc. 144, 2410–2416 (1997).
Wang, C. S., Wu, G. T., Zhang, X. B., Qi, Z. F. & Li, W. Z. Lithium insertion in carbon–silicon composite materials produced by mechanical milling. J. Electrochem. Soc. 145, 2751–2758 (1998).
Yoshio, M., Kugino, S. & Dimov, N. Electrochemical behaviours of silicon based anode material. J. Power Sources 153, 375–379 (2006).
Zhang, Y. et al. Composite anode material of silicon/graphite/carbon nanotubes for Li-ion batteries. Electrochim. Acta 51, 4994–5000 (2006).
Dimov, N., Kugino, S. & Yoshio, A. Mixed silicon–graphite composites as anode material for lithium ion batteries influence of preparation conditions on the properties of the material. J. Power Sources 136, 108–114 (2004).
Liu, Y. et al. Morphology-stable silicon-based composite for Li-intercalation. Solid State Ion. 168, 61–68 (2004).
Lee, H. Y. & Lee, S. M. Carbon-coated nano-Si dispersed oxides/graphite composites as anode material for lithium ion batteries. Electrochem. Commun. 6, 465–469 (2004).
Larcher, D. et al. Si-containing disordered carbons prepared by pyrolysis of pitch polysilane blends: Effect of oxygen and sulfur. Solid State Ion. 122, 71–83 (1999).
Timmons, A. et al. Studies of Si1−xCx electrode materials prepared by high-energy mechanical milling and combinatorial sputter deposition. J. Electrochem. Soc. 154, A865–A874 (2007).
Kasavajjula, U., Wang, C. S. & Appleby, A. J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 163, 1003–1039 (2007).
Yen, Y. C., Chao, S. C., Wu, H. C. & Wu, N. L. Study on solid–electrolyte-interphase of Si and C-coated Si electrodes in lithium cells. J. Electrochem. Soc. 156, A95–A102 (2009).
Baughman, R. H., Zakhidov, A. A. & de Heer, W. A. Carbon nanotubes—the route toward applications. Science 297, 787–792 (2002).
Huggins, R. A. & Nix, W. D. Decrepitation model for capacity loss during cycling of alloys in rechargeable electrochemical systems. Ionics 6, 57–63 (2000).
Ji, X. L., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nature Mater. 8, 500–506 (2009).
Whitesides, G. M. & Grzybowski, B. Self-assembly at all scales. Science 295, 2418–2421 (2002).
Boal, A. K. et al. Self-assembly of nanoparticles into structured spherical and network aggregates. Nature 404, 746–748 (2000).
Philp, D. & Stoddart, J. F. Self-assembly in natural and unnatural systems. Angew. Chem. Int. Edn 35, 1155–1196 (1996).
Iveson, S. M., Litster, J. D., Hapgood, K. & Ennis, B. J. Nucleation, growth and breakage phenomena in agitated wet granulation processes: A review. Powder Technol. 117, 3–39 (2001).
Nijhawan, S. et al. An experimental and numerical study of particle nucleation and growth during low-pressure thermal decomposition of silane. J. Aerosol. Sci. 34, 691–711 (2003).
Wissler, M. Graphite and carbon powders for electrochemical applications. J. Power Sources 156, 142–150 (2006).
Barsukov, I. V., Gallego, M. A. & Doninger, J. E. Novel materials for electrochemical power sources—introduction of PUREBLACK((R)) carbons. J. Power Sources 153, 288–299 (2006).
Portet, C., Yushin, G. & Gogotsi, Y. Electrochemical performance of carbon onions, nanodiamonds, carbon black and multiwalled nanotubes in electrical double layer capacitors. Carbon 45, 2511–2518 (2007).
Seo, A., Holm, P., Kristensen, H. G. & Schaefer, T. The preparation of agglomerates containing solid dispersions of diazepam by melt agglomeration in a high shear mixer. Int. J. Pharm. 259, 161–171 (2003).
Becker, A. & Huttinger, K. J. Chemistry and kinetics of chemical vapour deposition of pyrocarbon—III—pyrocarbon deposition from propylene and benzene in the low temperature regime. Carbon 36, 201–211 (1998).
Zakhidov, A. A. et al. Carbon structures with three-dimensional periodicity at optical wavelengths. Science 282, 897–901 (1998).
Vlasov, Y. A., Bo, X. Z., Sturm, J. C. & Norris, D. J. On-chip natural assembly of silicon photonic bandgap crystals. Nature 414, 289–293 (2001).
Kroutvar, M. et al. Optically programmable electron spin memory using semiconductor quantum dots. Nature 432, 81–84 (2004).
Keren, K., Berman, R. S., Buchstab, E., Sivan, U. & Braun, E. DNA-templated carbon nanotube field-effect transistor. Science 302, 1380–1382 (2003).
Jiang, C. Y., Markutsya, S., Pikus, Y. & Tsukruk, V. V. Freely suspended nanocomposite membranes as highly sensitive sensors. Nature Mater. 3, 721–728 (2004).
Rybin, M. V. et al. Fano resonance between Mie and Bragg scattering in photonic crystals. Phys. Rev. Lett. 103, 023901 (2009).
Rolison, D. R. Catalytic nanoarchitectures—the importance of nothing and the unimportance of periodicity. Science 299, 1698–1701 (2003).
Acknowledgements
The authors acknowledge the partial support from NASA through an SBIR grant NNX09CD29P 2008-1. We thank A. Alexeev, I. Luzinov, T. Fuller, B. Zdyrko, I. Barsukov, F. Henry, P. Wu, S. Boukhalfa, W. Lu, J. Benson and S. Gillain for valuable discussions or experimental assistance.
Author information
Authors and Affiliations
Contributions
A.M. carried out experiments, analysed and discussed data and wrote the paper; P.D. carried out experiments; B.H. carried out experiments, discussed data and wrote the paper; A.K. carried out experiments; J.A. discussed data and provided technical support; G.Y. conceived, designed and carried out experiments, analysed and discussed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. S1
Supplementary Information (PDF 585 kb)
Rights and permissions
About this article
Cite this article
Magasinski, A., Dixon, P., Hertzberg, B. et al. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nature Mater 9, 353–358 (2010). https://doi.org/10.1038/nmat2725
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2725
This article is cited by
-
High voltage electrolytes for lithium-ion batteries with micro-sized silicon anodes
Nature Communications (2024)
-
Carbon-coated disproportionated SiO composite as anode materials for lithium-ion batteries
Journal of Applied Electrochemistry (2024)
-
Formation of hierarchically ordered structures in conductive polymers to enhance the performances of lithium-ion batteries
Nature Energy (2023)
-
Encapsulating Si nanoparticles in multi-shell hollow spheres: An effective approach to boost the cyclability
Science China Materials (2023)
-
Designing of 3D porous silicon/carbon complex anode based on metal-organic frameworks for lithium-ion battery
Carbon Letters (2023)