Abstract
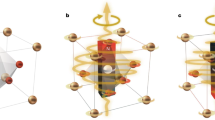
Nanomagnetic materials offer exciting avenues for probing cell mechanics and activating mechanosensitive ion channels, as well as for advancing cancer therapies. Most experimental works so far have used superparamagnetic materials. This report describes a first approach based on interfacing cells with lithographically defined microdiscs that possess a spin-vortex ground state. When an alternating magnetic field is applied the microdisc vortices shift, creating an oscillation, which transmits a mechanical force to the cell. Because reduced sensitivity of cancer cells toward apoptosis leads to inappropriate cell survival and malignant progression, selective induction of apoptosis is of great importance for the anticancer therapeutic strategies. We show that the spin-vortex-mediated stimulus creates two dramatic effects: compromised integrity of the cellular membrane, and initiation of programmed cell death. A low-frequency field of a few tens of hertz applied for only ten minutes was sufficient to achieve ∼90% cancer-cell destruction in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
13 January 2010
In the version of this Article originally published, the following sentence in the caption of Fig. 5 should have been written as: “Images of negative control (b,c) and MD-mAb-functionalized cells subjected to 20 Hz–90 Oe a.c. fields for 10 min and TUNEL stained 4 h after the magnetic-field exposure (d,e)” This has been corrected in all versions of this Article.
References
Chappert, C., Fert, A. & Van Dau, F. N. The emergence of spin electronics in data storage. Nature Mater. 6, 813–823 (2007).
Bruns, O. T. et al. Real-time magnetic resonance imaging and quantification of lipoprotein metabolism in vivo using nanocrystals. Nature Nanotech. 4, 193–201 (2009).
Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nature Rev. Cancer 5, 161–171 (2005).
Pankhurst, Q. A., Connolly, J., Jones, S. K. & Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D 36, R167–R175 (2003).
Hergt, R., Dutz, S., Muller, R. & Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter. 18, S2919–S2923 (2006).
Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nature Nanotech. 3, 139–148 (2008).
Mannix, R. J. et al. Nanomagnetic actuation of receptor-mediated signal transduction. Nature Nanotech. 3, 36–40 (2007).
Wang, N., Butler, J. P. & Ingber, D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 (1993).
Jun, Y.-W., Seo, J.-W. & Cheon, J. Nanoscaling laws of magnetic nanoparticles and their applicability in biomedical science. Acc. Chem. Res. 41, 179–186 (2008).
Cheon, J. & Lee, J.-H. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc. Chem. Res. 41, 1630–1635 (2008).
Cowburn, R. P., Koltsov, D. K., Adeyeye, A. O. & Welland, M. E. Single-domain circular nanomagnets. Phys. Rev. Lett. 83, 1042–1045 (1999).
Shinjo, T., Okuno, T., Hassdorf, R., Shigeto, K. & Ono, T. Magnetic vortex core observation in circular dots of permalloy. Science 289, 930–933 (2000).
Wachowiak, A. et al. Direct observation of internal spin structure of magnetic vortex cores. Science 298, 577–580 (2002).
Buchanan, K. S. et al. Soliton-pair dynamics in patterned ferromagnetic ellipses. Nature Phys. 1, 172–176 (2005).
Ade, H. & Stoll, H. Near-edge X-ray absorption fine-structure microscopy of organic and magnetic materials. Nature Mater. 8, 281–290 (2009).
Novosad, V. et al. Effect of interdot magnetostatic interaction on magnetization reversal in circular dot arrays. Phys. Rev. B 65, 060402 (2002).
Rozhkova, E. A. et al. Ferromagnetic microdisks as carriers for biomedical applications. J. Appl. Phys. 105, 07B306 (2009).
Martin, J. E., Hill, K. M. & Tigges, C. P. Magnetic-field-induced optical transmittance in colloidal suspensions. Phys. Rev. E 59, 5676–5692 (1999).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano-bio interface. Nature Mater. 8, 543–557 (2009).
Da, K., Shiyama, K., Naka, R., Hiyama, A. & Anishi, T. GFAP-positive human glioma cell lines: No. 10, no. 11. Hum. Cell 3, 251–256 (1990).
Debinski, W., Gibo, D., Hulet, S., Connor, J. & Gillespie, G. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Cancer Res. 5, 985–990 (1999).
Kawakami, K., Kawakami, M., Snoy, P. J., Husain, S. R. & Puri, R. K. In vivo overexpression of IL-13 receptor {alpha}2 chain inhibits tumorigenicity of human breast and pancreatic tumors in immunodeficient mice. J. Exp. Med. 194, 1743–1754 (2001).
Rozhkova, E. A. et al. A high-performance nanobio photocatalyst for targeted brain cancer therapy. Nano Lett. 9, 3337–3342 (2009).
Sen, S., Subramanian, S. & Discher, D. E. Indentation and adhesive probing of a cell membrane with AFM: Theoretical model and experiments. Biophys. J. 89, 3203–3213 (2005).
Afrin, R., Yamada, T. & Ikai, A. Analysis of force curves obtained on the live cell membrane using chemically modified AFM probes. Ultramicroscopy 100, 187–195 (2004).
Muller, D., Helenius, J., Alsteens, D. & Dufrêne, Y. Force probing surfaces of living cells to molecular resolution. Nature Chem. Biol. 5, 383–391 (2009).
Mpoke, S. S. & Wolfe, J. Differential staining of apoptotic nuclei in living cells: Application to macronuclear elimination in tetrahymena. J. Histochem. Cytochem. 45, 675–684 (1997).
Yakovlev, A. G. et al. Role of DNAS1L3 in Ca2+- and Mg2+-dependent cleavage of DNA into oligonucleosomal and high molecular mass fragments. Nucl. Acids Res. 27, 1999–2005 (1999).
Mattson, M. P. & Chan, S. L. Calcium orchestrates apoptosis. Nature Cell Biol. 5, 1041–1043 (2003).
Clapham, D. E. Calcium signaling. Cell 80, 259–268 (1995).
Duchen, M. R. Mitochondria and calcium: From cell signalling to cell death. J. Phys. 529, 57–68 (2000).
Boehning, D. et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nature Cell Biol. 5, 1051–1061 (2003).
Martinac, B. Mechanosensitive ion channels: Molecules of mechanotransduction. J. Cell Sci. 117, 2449–2460 (2004).
Guharay, F. & Sachs, F. J. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. Physiol. (London) 352, 685–701 (1984).
Diamond, S. L., Sachs, F. & Sigurdson, W. J. Mechanically induced mobilization in cultured endothelial cells is dependent on actin and phospholipase. Arterioscler. Thromb. Vasc. Biol. 14, 2000–2006 (1994).
Adachi, T., Sato, K. & Tomita, Y. Directional dependence of osteoblastic calcium response to mechanical stimuli. Biomech. Model Mechanobiol. 2, 73–82 (2003).
Hergt, R. & Dutz, S. Magnetic particle hyperthermia—biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 311, 187–192 (2007).
Goya, G. F., Grazu, V. & Ibarra, M. R. Magnetic nanoparticles for cancer therapy. Current Nanosci. 4, 1–16 (2008).
Gupta, A. K. & Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26, 3995–4021 (2005).
Acknowledgements
We thank D. Clapham, R. Hergt, J. Dobson and A. Datesman for valuable suggestions and critical reading of the manuscript. We also thank J. Pearson for help with developing the magnetic-field induction set-up, R. Divan for discussing the microfabrication strategies, and V. Bindokas for technical assistance in Ca imaging at the UC Biological Sciences Division Light Microscopy Core Facility. Work at Argonne and its Center for Nanoscale Materials and Electron Microscopy Center is supported by the US Department of Energy Office of Science, Basic Energy Sciences, under contract No DE-AC02-06CH11357. Work at the University of Chicago is supported by the National Cancer Institute (R01-CA122930), the National Institute of Neurological Disorders and Stroke (K08-NS046430), the Alliance for Cancer Gene Therapy Young Investigator Award and the American Cancer Society (RSG-07-276-01-MGO).
Author information
Authors and Affiliations
Contributions
V.N. and E.A.R. conceived the experimental idea. M.S.L. advanced the conceptual design for the glioma cell targeting. I.V.U. performed in vitro and cell cytotoxicity studies. D.-H.K. and V.N. fabricated the magnetic microdiscs and carried out the magnetic characterization and micromagnetic modelling. D.-H.K. and E.A.R. ran the biofunctionalization experiments. D.-H.K. carried out atomic force and optical microscopy characterizations. E.A.R. and I.V.U. designed and analysed the intracellular Ca imaging experiments. D.-H.K., E.A.R., I.V.U., T.R., S.D.B., M.S.L. and V.N. analysed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2299 kb)
Supplementary Information
Supplementary Movie (WMV 1646 kb)
Rights and permissions
About this article
Cite this article
Kim, DH., Rozhkova, E., Ulasov, I. et al. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nature Mater 9, 165–171 (2010). https://doi.org/10.1038/nmat2591
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2591
This article is cited by
-
Elucidating Mechanotransduction Processes During Magnetomechanical Neuromodulation Mediated by Magnetic Nanodiscs
Cellular and Molecular Bioengineering (2023)
-
Oxygen and hydrogen peroxide self-supplying magnetic nanoenzymes for cancer therapy through magneto-mechanical force, force-induced reactive oxygen species, chemodynamic effects, and cytotoxicity of Ca2+ ions
Nano Research (2023)
-
Nanoparticle-mediated cancer cell therapy: basic science to clinical applications
Cancer and Metastasis Reviews (2023)
-
Urchin-like magnetic microspheres for cancer therapy through synergistic effect of mechanical force, photothermal and photodynamic effects
Journal of Nanobiotechnology (2022)
-
Low frequency vibrating magnetic field-triggered magnetic microspheres with a nanoflagellum-like surface for cancer therapy
Journal of Nanobiotechnology (2022)