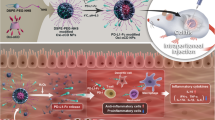
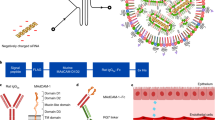
Abstract
Small interfering RNAs (siRNAs) directed against proinflammatory cytokines have the potential to treat numerous diseases associated with intestinal inflammation1; however, the side-effects caused by the systemic depletion of cytokines2,3,4 demands that the delivery of cytokine-targeted siRNAs be localized to diseased intestinal tissues. Although various delivery vehicles have been developed to orally deliver therapeutics to intestinal tissue5,6,7, none of these strategies has demonstrated the ability to protect siRNA from the harsh environment of the gastrointestinal tract and target its delivery to inflamed intestinal tissue. Here, we present a delivery vehicle for siRNA, termed thioketal nanoparticles (TKNs), that can localize orally delivered siRNA to sites of intestinal inflammation, and thus inhibit gene expression in inflamed intestinal tissue. TKNs are formulated from a polymer, poly-(1,4-phenyleneacetone dimethylene thioketal), that degrades selectively in response to reactive oxygen species (ROS). Therefore, when delivered orally, TKNs release siRNA in response to the abnormally high levels of ROS specific to sites of intestinal inflammation8,9,10. Using a murine model of ulcerative colitis, we demonstrate that orally administered TKNs loaded with siRNA against the proinflammatory cytokine tumour necrosis factor-alpha (TNF-α) diminish TNF-α messenger RNA levels in the colon and protect mice from ulcerative colitis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Peer, D., Park, E. J., Morishita, Y., Carman, C. V. & Shimaoka, M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319, 627–630 (2008).
Wolfe, F., Michaud, K., Anderson, J. & Urbansky, K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 50, 372–379 (2004).
Reddy, J. G. & Loftus, E. V. Jr Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol Clin. North Am. 35, 837–855 (2006).
Heraganahally, S. S. et al. Pulmonary toxicity associated with infliximab therapy for ulcerative colitis. Int. Med. J. 39, 629–630 (2009).
Aouadi, M. et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 458, 1180–1184 (2009).
Pertuit, D. et al. 5-amino salicylic acid bound nanoparticles for the therapy of inflammatory bowel disease. J. Control. Release. 123, 211–218 (2007).
Yamanaka, Y. J. & Leong, K. W. Engineering strategies to enhance nanoparticle-mediated oral delivery. J. Biomater. Sci. Polym. Ed. 19, 1549–1570 (2008).
Lih-Brody, L. et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 41, 2078–2086 (1996).
Kountouras, J., Chatzopoulos, D. & Zavos, C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology 48, 743–751 (2001).
Simmonds, N. J. et al. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology 103, 186–196 (1992).
Sedghi, S. et al. Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut 34, 1191–1197 (1993).
Peng, Y. C. et al. Chemiluminescence assay of mucosal reactive oxygen species in gastric cancer, ulcer and antral mucosa. Hepatogastroenterology 55, 770–773 (2008).
Shukla, A. K., Verma, M. & Singh, K. N. Superoxide induced deprotection of 1,3-dithiolanes: A convenient method of dedithioacetalization. Indian J. Chem. B 43, 1748–1752 (2004).
Colonna, S., Gaggero, N., Carrea, G. & Pasta, P. Enantio and diastereoselectivity of cyclohexanone monooxygenase catalyzed oxidation of 1,3-dithioacetals. Tetrahedron-Asymmetr. 7, 565–570 (1996).
Mahida, Y. R., Wu, K. C. & Jewell, D. P. Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel-disease. Gut 30, 1362–1370 (1989).
Kontoyiannis, D., Pasparakis, M., Pizarro, T. T., Cominelli, F. & Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: Implications for joint and gut-associated immunopathologies. Immunity 10, 387–398 (1999).
Sorensen, D. R., Leirdal, M. & Sioud, M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J. Mol. Biol. 327, 761–766 (2003).
Leirdal, M. & Sioud, M. Gene silencing in mammalian cells by preformed small RNA duplexes. Biochem. Biophys. Res. Commun. 295, 744–748 (2002).
Murata, N., Takashima, Y., Toyoshima, K., Yamamoto, M. & Okada, H. Anti-tumour effects of anti-VEGF siRNA encapsulated with PLGA microspheres in mice. J. Control. Release. 126, 246–254 (2008).
Palliser, D. et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature 439, 89–94 (2006).
Akhtar, S. & Benter, I. F. Nonviral delivery of synthetic siRNAs in vivo. J. Clin. Invest. 117, 3623–3632 (2007).
Zhang, S., Zhao, B., Jiang, H., Wang, B. & Ma, B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J. Control. Release. 123, 1–10 (2007).
Thiele, L. et al. Evaluation of particle uptake in human blood monocyte-derived cells in vitro. Does phagocytosis activity of dendritic cells measure up with macrophages? J. Control. Release. 76, 59–71 (2001).
Hariharan, S. et al. Design of estradiol loaded PLGA nanoparticulate formulations: A potential oral delivery system for hormone therapy. Pharm. Res. 23, 184–195 (2006).
Desai, M. P., Labhasetwar, V., Amidon, G. L. & Levy, R. J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 13, 1838–1845 (1996).
Lamprecht, A., Schafer, U. & Lehr, C. M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 18, 788–793 (2001).
Yan, Y. et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulphate induced colitis. PLoS One 4, e6073 (2009).
Wirtz, S., Neufert, C., Weigmann, B. & Neurath, M. F. Chemically induced mouse models of intestinal inflammation. Nature Protoc. 2, 541–546 (2007).
Acknowledgements
This project was financially supported by the Georgia Tech/Emory Center for the Engineering of Living Tissues (funded by NSF-EEC-9731643) (N.M.), NSF-BES-0546962 Career Award (N.M.), NIH UO1 HL80711-01 (N.M.), NIH R21 EB006418 (N.M.), NIH RO1 HL096796-01 (N.M.), NIH RO1-DK-071594 (D.M.) and NIH RO1-DK-064711 (S.V.S.). D.S.W. is supported by the Center for Drug Design, Development and Delivery at the Georgia Institute of Technology and the NIH Cellular and Tissue Engineering Training Grant T32 GM08433. G.D. is supported by a research fellowship award from the Crohn’s Colitis Foundation of America.
Author information
Authors and Affiliations
Contributions
D.S.W. synthesized and characterized PPADT; formulated particles; designed, carried out and analysed experiments; and wrote the manuscript. G.D. designed, carried out and analysed experiments; and proof read the manuscript. L.W. carried out experiments. S.V.S. supervised the project. D.M. designed experiments; supervised the project; and proof read the manuscript. N.M. designed the synthetic strategy used to synthesize PPADT; supervised the project; and contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2227 kb)
Rights and permissions
About this article
Cite this article
Wilson, D., Dalmasso, G., Wang, L. et al. Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nature Mater 9, 923–928 (2010). https://doi.org/10.1038/nmat2859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2859
This article is cited by
-
Probing milk extracellular vesicles for intestinal delivery of RNA therapies
Journal of Nanobiotechnology (2023)
-
Oral Delivery of Nucleic Acid Therapies for Local and Systemic Action
Pharmaceutical Research (2023)
-
Recent advances of bioresponsive polymeric nanomedicine for cancer therapy
Nano Research (2023)
-
Hydrogel–metal-organic-framework hybrids mediated efficient oral delivery of siRNA for the treatment of ulcerative colitis
Journal of Nanobiotechnology (2022)
-
Cartilage targeting therapy with reactive oxygen species-responsive nanocarrier for osteoarthritis
Journal of Nanobiotechnology (2022)