Abstract
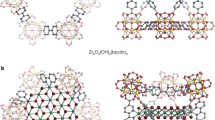
Cage structures exhibit inherent high symmetry and beauty, and both naturally occurring and synthetic molecular-scale cages have been discovered. Their characteristic high surface area and voids have led to their use as catalysts and catalyst supports, filtration media and gas storage materials1,2. Nanometre-scale cage structures have also been synthesized, notably noble-metal cube-shaped cages prepared by galvanic displacement with promising applications in drug delivery and catalysis3,4,5,6. Further functionality for nanostructures in general is provided by the concept of hybrid nanoparticles combining two disparate materials on the same system to achieve synergistic properties stemming from unusual material combinations7,8,9,10,11. We report the integration of the two powerful concepts of cages and hybrid nanoparticles. A previously unknown edge growth mechanism has led to a new type of cage-structured hybrid metal–semiconductor nanoparticle; a ruthenium cage was grown selectively on the edges of a faceted copper(I) sulphide nanocrystal, contrary to the more commonly observed facet and island growth modes of other hybrids7,12,13,14,15. The cage motif was extended by exploiting the open frame to achieve empty cages and cages containing other semiconductors. Such previously unknown nano-inorganic cage structures with variable cores and metal frames manifest new chemical, optical and electronic properties and demonstrate possibilities for uses in electrocatalysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469–472 (2002).
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 813–821 (2002).
Skrabalak, S. E., Au, L., Li, X. D. & Xia, Y. Facile synthesis of Ag nanocubes and Au nanocages. Nature Protoc. 2, 2182–2190 (2007).
Skrabalak, S. E. et al. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 41, 1587–1595 (2008).
Yavuz, M. S. et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nature Mater. 8, 935–939 (2009).
Zeng, J., Zhang, Q., Chen, J. Y. & Xia, Y. N. A comparison study of the catalytic properties of Au-based nanocages, nanoboxes, and nanoparticles. Nano Lett. 10, 30–35 (2010).
Costi, R., Saunders, A. E. & Banin, U. Colloidal hybrid nanostructures: A new type of functional materials. Angew. Chem. Int. Ed. 49, 4878–4897 (2010).
Mokari, T., Rothenberg, E., Popov, I., Costi, R. & Banin, U. Selective growth of metal tips onto semiconductor quantum rods and tetrapods. Science 304, 1787–1790 (2004).
Cozzoli, P. D., Pellegrino, T. & Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem. Soc. Rev. 35, 1195–1208 (2006).
Wetz, F. et al. Hybrid Co–Au nanorods: Controlling Au nucleation and location. Angew. Chem. Int. Ed. 46, 7079–7081 (2007).
Habas, S. E., Yang, P. D. & Mokari, T. Selective growth of metal and binary metal tips on CdS nanorods. J. Am. Chem. Soc. 130, 3294–3295 (2008).
Sadtler, B. et al. Selective facet reactivity during cation exchange in cadmium sulfide nanorods. J. Am. Chem. Soc. 131, 5285–5293 (2009).
Han, W. et al. Synthesis and shape-tailoring of copper sulfide/indium sulfide-based nanocrystals. J. Am. Chem. Soc. 130, 13152–13161 (2008).
Shi, W. L. et al. A general approach to binary and ternary hybrid nanocrystals. Nano Lett. 6, 875–881 (2006).
Menagen, G., Macdonald, J. E., Shemesh, Y., Popov, I. & Banin, U. Au growth on semiconductor nanorods: Photoinduced versus thermal growth mechanisms. J. Am. Chem. Soc. 131, 17406–17411 (2009).
Figuerola, A. et al. End-to-end assembly of shape-controlled nanocrystals via a nanowelding approach mediated by gold domains. Adv. Mater. 21, 550–554 (2009).
Maynadié, J. et al. Cobalt growth on the tips of CdSe nanorods. Angew. Chem. Int. Ed. 48, 1814–1817 (2009).
Zhao, N., Liu, K., Greener, J., Nie, Z. H. & Kumacheva, E. Close-packed superlattices of side-by-side assembled Au–CdSe nanorods. Nano Lett. 9, 3077–3081 (2009).
Choi, S. H. et al. Simple and generalized synthesis of semiconducting metal sulfide nanocrystals. Adv. Funct. Mater. 19, 1645–1649 (2009).
Joint Committee on Powder Diffraction Standards (JSPDS) cards employed for structural determination: hcp ruthenium: 03-065-1863, low chalcocite: 03-033-0490, djurleite: 00-034-0660, hexagonal CdS: 01-077-2306, PbS: 03-065-2935.
Zhao, F. H. et al. Controlled growth of Cu2S hexagonal microdisks and their optical properties. J. Phys. Chem. Solids 67, 1786–1791 (2006).
Midgley, P. A. & Dunin-Borkowski, R. E. Electron tomography and holography in materials science. Nature Mater. 8, 271–280 (2009).
Bar Sadan, M., Wolf, S. G. & Houben, L. Bright-field electron tomography of individual inorganic fullerene-like structures. Nanoscale 2, 423–428 (2010).
Zhao, Y. X. et al. Plasmonic Cu2−xS nanocrystals: Optical and structural properties of copper-deficient copper(I) sulfides. J. Am. Chem. Soc. 131, 4253–4261 (2009).
Wu, Y., Wadia, C., Ma, W. L., Sadtler, B. & Alivisatos, A. P. Synthesis and photovoltaic application of copper(I) sulfide nanocrystals. Nano Lett. 8, 2551–2555 (2008).
Talapin, D. V., Yu, H., Shevchenko, E. V., Lobo, A. & Murray, C. B. Synthesis of colloidal PbSe/PbS core–shell nanowires and PbS/Au nanowire-nanocrystal heterostructures. J. Phys. Chem. C 111, 14049–14054 (2007).
Mokari, T., Sztrum, C. G., Salant, A., Rabani, E. & Banin, U. Formation of asymmetric one-sided metal-tipped semiconductor nanocrystal dots and rods. Nature Mater. 4, 855–863 (2005).
Myung, Y. et al. Nonenzymatic amperometric glucose sensing of platinum, copper sulfide, and tin oxide nanoparticle-carbon nanotube hybrid nanostructures. J. Phys. Chem. C 113, 1251–1259 (2009).
Luther, J. M., Zheng, H. M., Sadtler, B. & Alivisatos, A. P. Synthesis of PbS nanorods and other ionic nanocrystals of complex morphology by sequential cation exchange reactions. J. Am. Chem. Soc. 131, 16851–16857 (2009).
Connor, S. T., Hsu, C. M., Weil, B. D., Aloni, S. & Cui, Y. Phase transformation of biphasic Cu2S-CuInS2 to monophasic CuInS2 nanorods. J. Am. Chem. Soc. 131, 4962–4966 (2009).
Acknowledgements
Partial financial support by the Israel Science Foundation (grant 972/08), and the ERC grant DCENSY is acknowledged. U.B. thanks the Alfred and Erica Larisch Memorial Chair in Solar Energy. M.B.S. thanks the Minerva Fellowship program funded by the German Federal Ministry for Education and Research and the Sara Lee Schupf Postdoctoral Fellowship. The authors also thank D. Mandler for use of electrochemisty instrumentation.
Author information
Authors and Affiliations
Contributions
J.E.M. and U.B. designed the experiments and wrote the manuscript. J.E.M. carried out the experiments, materials characterization and analysis. I.P. assisted with HAADF-STEM and energy-dispersive X-ray spectroscopy measurements and provided commentary on the manuscript and materials analysis. M.B.S. carried out the tomography experiments and the analysis of its data and wrote parts of the manuscripts. L.H. wrote the tomographic processing software and assisted in the reconstruction, provided the aberration-corrected HAADF-STEM images and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Movie 1 (MOV 1567 kb)
Supplementary Information
Supplementary Movie 2 (MOV 2992 kb)
Rights and permissions
About this article
Cite this article
Macdonald, J., Bar Sadan, M., Houben, L. et al. Hybrid nanoscale inorganic cages. Nature Mater 9, 810–815 (2010). https://doi.org/10.1038/nmat2848
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2848
This article is cited by
-
Synthesis, Characterization, and Potential Applications of Transition Metal Nanoparticles
Journal of Inorganic and Organometallic Polymers and Materials (2020)
-
Versatile synthesis of yolk/shell hybrid nanocrystals via ion-exchange reactions for novel metal/semiconductor and semiconductor/semiconductor conformations
Nano Research (2017)
-
Understanding of the major reactions in solution synthesis of functional nanomaterials
Science China Materials (2016)
-
Rational synthesis and the structure-property relationships of nanoheterostructures: a combinative study of experiments and theory
NPG Asia Materials (2015)
-
Nanoscale noble metals with a hollow interior formed through inside-out diffusion of silver in solid-state core-shell nanoparticles
Nano Research (2015)