Abstract
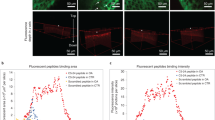
The extracellular matrix of dense, avascular tissues presents a barrier to entry for polymer-based therapeutics, such as drugs encapsulated within polymeric particles. Here, we present an approach by which polymer nanoparticles, sufficiently small to enter the matrix of the targeted tissue, here articular cartilage, are further modified with a biomolecular ligand for matrix binding. This combination of ultrasmall size and biomolecular binding converts the matrix from a barrier into a reservoir, resisting rapid release of the nanoparticles and clearance from the tissue site. Phage display of a peptide library was used to discover appropriate targeting ligands by biopanning on denuded cartilage. The ligand WYRGRL was selected in 94 of 96 clones sequenced after five rounds of biopanning and was demonstrated to bind to collagen II α1. Peptide-functionalized nanoparticles targeted articular cartilage up to 72-fold more than nanoparticles displaying a scrambled peptide sequence following intra-articular injection in the mouse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bae, Y. et al. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 16, 122–130 (2005).
Dreher, M. R. et al. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl Cancer Inst. 98, 335–344 (2006).
Duncan, R. The dawning era of polymer therapeutics. Nature Rev. Drug. Discov. 2, 347–360 (2003).
Shiah, J. J., Sun, Y., Peterson, C. M. & Kopecek, J. Biodistribution of free and N-(2-hydroxypropyl)methacrylamide copolymer-bound mesochlorin e(6) and adriamycin in nude mice bearing human ovarian carcinoma OVCAR-3 xenografts. J. Control Release 61, 145–157 (1999).
Owen, S. G., Francis, H. W. & Roberts, M. S. Disappearance kinetics of solutes from synovial fluid after intra-articular injection. Br. J. Clin. Pharmacol. 38, 349–355 (1994).
Comper, W. D. in Cartilage: Molecular Aspects (eds Hall, B. & Newman, S.) 59–96 (CRC Press, Boston, 1991).
Torzilli, P. A., Arduino, J. M., Gregory, J. D. & Bansal, M. Effect of proteoglycan removal on solute mobility in articular cartilage. J. Biomech. 30, 895–902 (1997).
Arap, W. et al. Steps toward mapping the human vasculature by phage display. Nature Med. 8, 121–127 (2002).
Kay, B. K., Kasanov, J. & Yamabhai, M. Screening phage-displayed combinatorial peptide libraries. Methods 24, 240–246 (2001).
Kolonin, M. G. et al. Synchronous selection of homing peptides for multiple tissues by in vivo phage display. FASEB J. 20, 979–981 (2006).
Pasqualini, R. & Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 380, 364–366 (1996).
Smith, G. P. & Petrenko, V. A. Phage display. Chem. Rev. 97, 391–410 (1997).
Cherney, R. J. et al. Potent and selective aggrecanase inhibitors containing cyclic P1 substituents. Bioorg. Med. Chem. Lett. 13, 1297–1300 (2003).
Noe, M. C. et al. Discovery of 3,3-dimethyl-5-hydroxypipecolic hydroxamate-based inhibitors of aggrecanase and MMP-13. Bioorg. Med. Chem. Lett. 15, 2808–2811 (2005).
Noe, M. C. et al. Discovery of 3-OH-3-methylpipecolic hydroxamates: Potent orally active inhibitors of aggrecanase and MMP-13. Bioorg. Med. Chem. Lett. 15, 3385–3388 (2005).
Noe, M. C. et al. 3-Hydroxy-4-arylsulfonyltetrahydropyranyl-3-hydroxamic acids are novel inhibitors of MMP-13 and aggrecanase. Bioorg. Med. Chem. Lett. 14, 4727–4730 (2004).
Yao, W. et al. Design and synthesis of a series of (2R)-N(4)-hydroxy-2-(3-hydroxybenzyl)-N(1)-[(1S,2R)-2-hydroxy-2, 3-dihydro-1H-inden-1-yl]butanediamide derivatives as potent, selective, and orally bioavailable aggrecanase inhibitors. J. Med. Chem. 44, 3347–3350 (2001).
Rehor, A., Hubbell, J. A. & Tirelli, N. Oxidation-sensitive polymeric nanoparticles. Langmuir 21, 411–417 (2005).
Rehor, A. Poly(propylene sulfide) Nanoparticles as Drug Carriers. Thesis, Swiss Federal Institute of Technology (2005).
Wieland, H. A., Michaelis, M., Kirschbaum, B. J. & Rudolphi, K. A. Osteoarthritis—an untreatable disease? Nature Rev. Drug. Discov. 4, 331–344 (2005).
Zacher, A. N. 3rd, Stock, C. A., Golden, J. W. 2nd & Smith, G. P. A new filamentous phage cloning vector: fd-tet. Gene 9, 127–140 (1980).
Lutolf, M. P. & Hubbell, J. A. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 4, 713–722 (2003).
Boder, E. T. & Wittrup, K. D. Yeast surface display for screening combinatorial polypeptide libraries. Nature Biotechnol. 15, 553–557 (1997).
Xu, L. et al. Directed evolution of high-affinity antibody mimics using mRNA display. Chem. Biol. 9, 933–942 (2002).
Lam, K. S., Lebl, M. & Krchnak, V. The “one-bead-one-compound” combinatorial library method. Chem. Rev. 97, 411–448 (1997).
Madry, H., Cucchiarini, M., Terwilliger, E. F. & Trippel, S. B. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum. Gene Ther. 14, 393–402 (2003).
Gao, H., Shi, W. & Freund, L. B. Mechanics of receptor-mediated endocytosis. Proc. Natl Acad. Sci. USA 102, 9469–9474 (2005).
Lynch, I., Dawson, K. A. & Linse, S. Detecting cryptic epitopes created by nanoparticles. Sci. STKE 2006, pe14 (2006).
Nagayama, S. et al. Fetuin mediates hepatic uptake of negatively charged nanoparticles via scavenger receptor. Int. J. Pharm. 329, 192–198 (2007).
Guilak, F. et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann. NY Acad. Sci. 1068, 498–512 (2006).
Phillips, N. C., Thomas, D. P., Knight, C. G. & Dingle, J. T. Liposome-incorporated corticosteroids. II. Therapeutic activity in experimental arthritis. Ann. Rheum. Dis. 38, 553–557 (1979).
Shaw, I. H., Knight, C. G., Thomas, D. P., Phillips, N. C. & Dingle, J. T. Liposome-incorporated corticosteroids: I. The interaction of liposomal cortisol palmitate with inflammatory synovial membrane. Br. J. Exp. Pathol. 60, 142–150 (1979).
Ratcliffe, J. H., Hunneyball, I. M., Smith, A., Wilson, C. G. & Davis, S. S. Preparation and evaluation of biodegradable polymeric systems for the intra-articular delivery of drugs. J. Pharm. Pharmacol. 36, 431–436 (1984).
Ratcliffe, J. H., Hunneyball, I. M., Wilson, C. G., Smith, A. & Davis, S. S. Albumin microspheres for intra-articular drug delivery: Investigation of their retention in normal and arthritic knee joints of rabbits. J. Pharm. Pharmacol. 39, 290–295 (1987).
Tuncay, M. et al. In vitro and in vivo evaluation of diclofenac sodium loaded albumin microspheres. J. Microencapsul. 17, 145–155 (2000).
Horisawa, E. et al. Prolonged anti-inflammatory action of DL-lactide/glycolide copolymer nanospheres containing betamethasone sodium phosphate for an intra-articular delivery system in antigen-induced arthritic rabbit. Pharm. Res. 19, 403–410 (2002).
Horisawa, E. et al. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm. Res. 19, 132–139 (2002).
Quinn, T. M., Morel, V. & Meister, J. J. Static compression of articular cartilage can reduce solute diffusivity and partitioning: Implications for the chondrocyte biological response. J. Biomech. 34, 1463–1469 (2001
Quinn, T. M., Kocian, P. & Meister, J. J. tatic compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch. Biochem. Biophys. 384, 327–334 (2000
Acknowledgements
The authors thank V. Garea and M. Pasquier for the help with the animal work and histology, A. Rehor for help with and discussions on initial nanoparticle synthesis, R. Schoenmakers for peptide synthesis, A. van der Vlies for obtaining 1H-NMR spectra, and especially the BIOP core facility for their support in establishing the imaging and quantification protocol and the proteomics core facility for running mass spectrometry analyses, and M. Lütolf for his help and suggestions in preparing the manuscript. DAR was supported by a grant from the Hans-Neuenschwander-Stiftung, Berne.
Author information
Authors and Affiliations
Contributions
The experiments have been designed by D.A.R. and J.A.H. and carried out by D.A.R. H.B. introduced phage display and aided in establishing initial protocols. C.P.O. assisted in the affinity purification. The manuscript was written by D.A.R. and J.A.H. Principal investigator is J.A.H.
Corresponding author
Ethics declarations
Competing interests
The EPFL has filed an application for a patent on the technology described in this manuscript, of which D.A.R. and J.A.H. are inventors.
Supplementary information
Supplementary Information, Table S1
Supplementary Table S1 and Supplementary figures S1-S3 (PDF 343 kb)
Rights and permissions
About this article
Cite this article
Rothenfluh, D., Bermudez, H., O’Neil, C. et al. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nature Mater 7, 248–254 (2008). https://doi.org/10.1038/nmat2116
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2116
This article is cited by
-
Injectable photocrosslinking spherical hydrogel-encapsulated targeting peptide-modified engineered exosomes for osteoarthritis therapy
Journal of Nanobiotechnology (2023)
-
Bioinspired surface functionalization of biodegradable mesoporous silica nanoparticles for enhanced lubrication and drug release
Friction (2023)
-
Modification of mesenchymal stem cells for cartilage-targeted therapy
Journal of Translational Medicine (2022)
-
Hierarchical functional nanoparticles boost osteoarthritis therapy by utilizing joint-resident mesenchymal stem cells
Journal of Nanobiotechnology (2022)
-
Collagen-binding peptides for the enhanced imaging, lubrication and regeneration of osteoarthritic articular cartilage
Nature Biomedical Engineering (2022)