Abstract
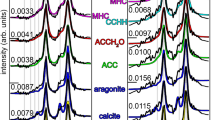
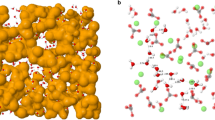
Composite biogenic materials produced by organisms have a complicated design on a nanometre scale1,2,3. An outstanding example of organic–inorganic composites is provided by mollusc seashells, whose superior mechanical properties are due to their multi-level crystalline hierarchy4,5 and the presence of a small amount (0.1–5 wt%)6 of organic molecules2,7. The presence of organic molecules, among other characteristics, can influence the coherence length for X-ray scattering in biogenic crystals8,9,10. Here we show the results of synchrotron high-resolution X-ray powder diffraction measurements in biogenic and non-biogenic (geological) aragonite crystals. On applying the Rietveld refinement procedure to the high-resolution diffraction spectra, we were able to extract the aragonite lattice parameters with an accuracy of 10 p.p.m. As a result, we found anisotropic lattice distortions in biogenic aragonite relative to the geological sample, maximum distortion being 0.1% along the c axis of the orthorhombic unit cell. The organic molecules could be a source of these structural distortions in biogenic crystals. This finding may be important to the general understanding of the biomineralization process and the development of bio-inspired 'smart' materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lowenstam, H. A. & Weiner, S. On Biomineralization (Oxford Univ. Press, New York, 1989).
Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials (Oxford Univ. Press, Oxford, 2001).
Weiner, S. & Addadi, L. Design strategies in mineralized biological materials. J. Mater. Chem. 7, 689–702 (1997).
Kamat, S., Su, X., Ballarini, R. & Heuer, A. H. Structural basis for the fracture toughness of the shell of the conch Strombus gigas. Nature 405, 1036–1040 (2000).
Pokroy, B. & Zolotoyabko, E. Microstructure of natural plywood-like ceramics: a study by high-resolution electron microscopy and energy-variable X-ray diffraction. J. Mater. Chem. 13, 682–688 (2003).
Hare, P. E. & Abelson, P. H. Amino acid composition of some calcified proteins. Ybk Carnegie Inst. Washington 65, 223–234 (1965).
Smith, B. L. et al. Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature 399, 761–763 (1999).
Berman, A., Addadi, L. & Weiner, S. Interactions of sea-urchin skeleton macromolecules with growing calcite crystals—a study of intracrystalline proteins. Nature 331, 546–548 (1988).
Berman, A. et al. Biological control of crystal texture: a widespread strategy for adapting crystal properties to function. Science 259, 776–779 (1993).
Aizenberg, J. et al. Biologically induced reduction in symmetry: a study of crystal texture of calcitic sponge spicules. Chem. Eur. J. 1, 414–422 (1995).
Okumura, M. & Kitano, Y. Coprecipitation of alkali-metal ions with calcium carbonate. Geochim. Cosmochim. Acta 50, 49–58 (1986).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Labotka, T. C., Cole, D. R. & Riciputi, L. R. Diffusion of C and O in calcite at 100 MPa. Am. Mineral. 85, 488–494 (2000).
Coradin, T., Coupe, A. & Livage, J. Intercalation of biomolecules in the MnPS3 layered phase. J. Mater. Chem. 13, 705–707 (2003).
Whilton, N. T., Vickers, P. J. & Mann, S. Bioinorganic clays: synthesis and characterization of amino- and polyamino acid intercalated layered double hydroxides. J. Mater. Chem. 7, 1623–1629 (1997).
Stupp, S. I. & Braun, P. V. Molecular manipulation of microstructures: Biomaterials, ceramics, and semiconductors. Science 277, 1242–1248 (1997).
Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 192, 55–69 (1993).
Thompson, P., Cox, D. E. & Hastings, J. B. Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3 . J. Appl. Crystallogr. 20, 79–83 (1987).
Acknowledgements
This work was supported by the Israel Science Foundation founded by the Israel Academy of Science and Humanities (grant no. 15/03-12.6). We thank S. Weiner (Weizmann Institute of Science, Rehovot) for discussions of different aspects of biomineralization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Pokroy, B., Quintana, J., Caspi, E. et al. Anisotropic lattice distortions in biogenic aragonite. Nature Mater 3, 900–902 (2004). https://doi.org/10.1038/nmat1263
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1263
This article is cited by
-
Rapid grain boundary diffusion in foraminifera tests biases paleotemperature records
Communications Earth & Environment (2023)
-
A Strong Inhibitory Effect of Microbe-Induced Mineralization on Corrosion on Steel Surfaces
Journal of Materials Engineering and Performance (2023)
-
A glance at the chemistry of calicoblastic epithelioma in Acropora valida
Thalassas: An International Journal of Marine Sciences (2023)
-
Crystallographic and chemical signatures in coral skeletal aragonite
Coral Reefs (2022)
-
Strategies for simultaneous strengthening and toughening via nanoscopic intracrystalline defects in a biogenic ceramic
Nature Communications (2020)