Abstract
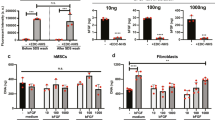
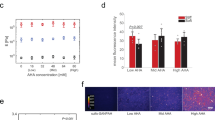
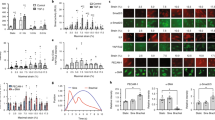
Expansion on stiff culture substrates activates pro-fibrotic cell programs that are retained by mechanical memory. Here, we show that priming on physiologically soft silicone substrates suppresses fibrogenesis and desensitizes mesenchymal stem cells (MSCs) against subsequent mechanical activation in vitro and in vivo, and identify the microRNA miR-21 as a long-term memory keeper of the fibrogenic program in MSCs. During stiff priming, miR-21 levels were gradually increased by continued regulation through the acutely mechanosensitive myocardin-related transcription factor-A (MRTF-A/MLK-1) and remained high over 2 weeks after removal of the mechanical stimulus. Knocking down miR-21 once by the end of the stiff-priming period was sufficient to erase the mechanical memory and sensitize MSCs to subsequent exposure to soft substrates. Soft priming and erasing mechanical memory following cell culture expansion protects MSCs from fibrogenesis in the host wound environment and increases the chances for success of MSC therapy in tissue-repair applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Balestrini, J. L., Chaudhry, S., Sarrazy, V., Koehler, A. & Hinz, B. The mechanical memory of lung myofibroblasts. Integr. Biol. 4, 410–421 (2012).
Yang, C., Tibbitt, M. W., Basta, L. & Anseth, K. S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652 (2014).
Jackson, W. M., Nesti, L. J. & Tuan, R. S. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem. Cell Res. Ther. 3, 20 (2012).
Leclerc, T. et al. Cell therapy of burns. Cell Prolif. 44, 48–54 (2011).
Sorrell, J. M. & Caplan, A. I. Topical delivery of mesenchymal stem cells and their function in wounds. Stem. Cell Res. Ther. 1, 30 (2010).
Hinz, B. et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am. J. Pathol. 180, 1340–1355 (2012).
Hinz, B. The myofibroblast: paradigm for a mechanically active cell. J. Biomech. 43, 146–155 (2010).
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R. A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363 (2002).
Majd, H. et al. A novel method of dynamic culture surface expansion improves mesenchymal stem cell proliferation and phenotype. Stem Cells 27, 200–209 (2009).
Talele, N. P., Fradette, J., Davies, J. E., Kapus, A. & Hinz, B. Expression of α-smooth muscle actin determines the fate of mesenchymal stromal cells. Stem Cell Rep. 4, 1016–1030 (2015).
Liu, F. et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 190, 693–706 (2010).
Achterberg, V. F. et al. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Invest. Dermatol. 134, 1862–1872 (2014).
Goffin, J. M. et al. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 172, 259–268 (2006).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004).
Jiang, X., Tsitsiou, E., Herrick, S. E. & Lindsay, M. A. MicroRNAs and the regulation of fibrosis. FEBS J. 277, 2015–2021 (2010).
Chau, B. N. & Brenner, D. A. What goes up must come down: the emerging role of microRNA in fibrosis. Hepatology 53, 4–6 (2011).
Mann, J. & Mann, D. A. Epigenetic regulation of wound healing and fibrosis. Curr. Opin. Rheumatol. 25, 101–107 (2013).
Bowen, T., Jenkins, R. H. & Fraser, D. J. MicroRNAs, transforming growth factor β-1, and tissue fibrosis. J. Pathol. 229, 274–285 (2013).
Pottier, N., Cauffiez, C., Perrais, M., Barbry, P. & Mari, B. FibromiRs: translating molecular discoveries into new anti-fibrotic drugs. Trends Pharmacol. Sci. 35, 119–126 (2014).
Babalola, O., Mamalis, A., Lev-Tov, H. & Jagdeo, J. The role of microRNAs in skin fibrosis. Arch. Dermatol. Res. 305, 763–776 (2013).
Fujita, S. et al. miR-21 gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J. Mol. Biol. 378, 492–504 (2008).
Zhang, X., Azhar, G., Helms, S. A. & Wei, J. Y. Regulation of cardiac microRNAs by serum response factor. J. Biomed. Sci. 18, 15 (2011).
Crider, B. J., Risinger, G. M. Jr, Haaksma, C. J., Howard, E. W. & Tomasek, J. J. Myocardin-related transcription factors A and B are key regulators of TGF-β1-induced fibroblast to myofibroblast differentiation. J. Invest. Dermatol. 131, 2378–2385 (2011).
Scharenberg, M. A. et al. TGF-β-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J. Cell Sci. 127, 1079–1091 (2014).
Zhou, Y. et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J. Clin. Invest. 123, 1096–1108 (2013).
Small, E. M. et al. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ. Res. 107, 294–304 (2010).
Luchsinger, L. L., Patenaude, C. A., Smith, B. D. & Layne, M. D. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J. Biol. Chem. 286, 44116–44125 (2011).
Ho, C. Y., Jaalouk, D. E., Vartiainen, M. K. & Lammerding, J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497, 507–511 (2013).
Fan, L. et al. Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol. Biol. Cell 18, 1083–1097 (2007).
Esnault, C. et al. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 28, 943–958 (2014).
Cavarretta, E. & Condorelli, G. miR-21 and cardiac fibrosis: another brick in the wall? Eur. Heart J. 36, 2139–2141 (2015).
Huang, Y., He, Y. & Li, J. MicroRNA-21: a central regulator of fibrotic diseases via various targets. Curr. Pharm. Des. 21, 2236–2242 (2015).
Wang, T. et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am. J. Pathol. 181, 1911–1920 (2012).
Chen, Z., Dai, T., Chen, X., Tan, L. & Shi, C. Activation and regulation of the granulation tissue derived cells with stemness-related properties. Stem Cell Res. Ther. 6, 85 (2015).
Thum, T. & Lorenzen, J. M. Cardiac fibrosis revisited by microRNA therapeutics. Circulation 126, 800–802 (2012).
Chau, B. N. et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci. Transl. Med. 4, 121ra118 (2012).
Liu, G. et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207, 1589–1597 (2010).
Zhu, H. et al. MicroRNA-21 in scleroderma fibrosis and its function in TGF-β-regulated fibrosis-related genes expression. J. Clin. Immunol. 33, 1100–1109 (2013).
Liang, H. et al. A novel reciprocal loop between microRNA-21 and TGFβRIII is involved in cardiac fibrosis. Int. J. Biochem. Cell Biol. 44, 2152–2160 (2012).
Thum, T. et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984 (2008).
Yao, Q. et al. Micro-RNA-21 regulates TGF-β-induced myofibroblast differentiation by targeting PDCD4 in tumor-stroma interaction. Int. J. Cancer 128, 1783–1792 (2011).
Gong, C. et al. miR-21 induces myofibroblast differentiation and promotes the malignant progression of breast phyllodes tumors. Cancer Res. 74, 4341–4352 (2014).
Parikh, V. N. et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation 125, 1520–1532 (2012).
Clement, S., Hinz, B., Dugina, V., Gabbiani, G. & Chaponnier, C. The N-terminal Ac-EEED sequence plays a role in {α}-smooth-muscle actin incorporation into stress fibers. J. Cell Sci. 118, 1395–1404 (2005).
Hinz, B. Myofibroblasts. Exp. Eye Res. 142, 56–70 (2016).
Li, H., Yang, F., Wang, Z., Fu, Q. & Liang, A. MicroRNA-21 promotes osteogenic differentiation by targeting small mothers against decapentaplegic 7. Mol. Med. Rep. 12, 1561–1567 (2015).
Song, Q. et al. miR-21 synergizes with BMP9 in osteogenic differentiation by activating the BMP9/Smad signaling pathway in murine multilineage cells. Int. J. Mol. Med. 36, 1497–1506 (2015).
Trohatou, O. et al. Sox2 suppression by miR-21 governs human mesenchymal stem cell properties. Stem Cells Transl. Med. 3, 54–68 (2014).
Hinz, B., Mastrangelo, D., Iselin, C. E., Chaponnier, C. & Gabbiani, G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am. J. Pathol. 159, 1009–1020 (2001).
Davidson, J. M., Yu, F. & Opalenik, S. R. Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv. Wound Care 2, 142–148 (2013).
Hinz, B., Gabbiani, G. & Chaponnier, C. The NH2-terminal peptide of α-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J. Cell Biol. 157, 657–663 (2002).
Chan, W. L., Silberstein, J. & Hai, C. M. Mechanical strain memory in airway smooth muscle. Am. J. Physiol. 278, C895–C904 (2000).
Song, J. et al. Mechanical stretch modulates microRNA 21 expression, participating in proliferation and apoptosis in cultured human aortic smooth muscle cells. PLoS ONE 7, e47657 (2012).
Neth, P., Nazari-Jahantigh, M., Schober, A. & Weber, C. MicroRNAs in flow-dependent vascular remodelling. Cardiovasc. Res. 99, 294–303 (2013).
Zhou, J. et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-α in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc. Natl Acad. Sci. USA 108, 10355–10360 (2011).
Chen, L. J., Wei, S. Y. & Chiu, J. J. Mechanical regulation of epigenetics in vascular biology and pathobiology. J. Cell Mol. Med. 17, 437–448 (2013).
Liu, F. et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. 308, L344–L357 (2015).
Cui, Y. et al. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 6, 6333 (2015).
Halder, G., Dupont, S. & Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol Cell Biol. 13, 591–600 (2012).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Calvo, F. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637–646 (2013).
Varelas, X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141, 1614–1626 (2014).
Piccolo, S., Dupont, S. & Cordenonsi, M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94, 1287–1312 (2014).
Gantier, M. P. et al. Analysis of microRNA turnover in mammalian cells following Dicer1 ablation. Nucleic Acids Res. 39, 5692–5703 (2011).
Hinson, J. S., Medlin, M. D., Taylor, J. M. & Mack, C. P. Regulation of myocardin factor protein stability by the LIM-only protein FHL2. Am. J. Physiol. 295, H1067–H1075 (2008).
Tariki, M. et al. The Yes-associated protein controls the cell density regulation of Hedgehog signaling. Oncogenesis 3, e112 (2014).
Vigneron, A. M., Ludwig, R. L. & Vousden, K. H. Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev. 24, 2430–2439 (2010).
Dingal, P. C. et al. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat. Mater. 14, 951–960 (2015).
Escacena, N., Quesada-Hernandez, E., Capilla-Gonzalez, V., Soria, B. & Hmadcha, A. Bottlenecks in the efficient use of advanced therapy medicinal products based on mesenchymal stromal cells. Stem Cells Int. 2015, 895714 (2015).
Wu, Y., Wang, J., Scott, P. G. & Tredget, E. E. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 15, S18–S26 (2007).
Qi, Y. et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Invest. Dermatol. 134, 526–537 (2014).
Zhang, L. & Chan, C. Isolation and enrichment of rat mesenchymal stem cells (MSCs) and separation of single-colony derived MSCs. J. Vis. Exp. 22, 1852 (2010).
Hinz, B. Matrix mechanics and regulation of the fibroblast phenotype. Periodontol. 2000 63, 14–28 (2013).
Wipff, P. J. et al. The covalent attachment of adhesion molecules to silicone membranes for cell stretching applications. Biomaterials 30, 1781–1789 (2009).
Darby, I., Skalli, O. & Gabbiani, G. α-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab. Invest. 63, 21–29 (1990).
Sarrazy, V. et al. Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc. Res. 102, 407–417 (2014).
Vartiainen, M. K., Guettler, S., Larijani, B. & Treisman, R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752 (2007).
Acknowledgements
We are grateful to J. Lammerding (Cornell University, Ithaca, New York, USA) and M. K. Vartiainen (University of Helsinki, Helsinki, Finland) for providing full-length and truncated constructs of MRTF-A-GFP. This research was supported by grants to B.H. from the Canadian Institutes of Health Research CIHR (grants no. 210820, no. 286920 and no. 286720), the Collaborative Health Research Programme (CIHR/NSERC grants no. 1004005 and no. 413783), and the Canada Foundation for Innovation and Ontario Research Fund (CFI/ORF grant no. 26653) and grants to A.Kapus from the CIHR (MOP-106625 and 130463), the Canadian Foundation of Innovation and the Kidney Foundation of Canada. Data presented herein were further funded from the European Union’s Seventh Framework Program (FP7/2007-2013) under grant agreement no. 237946 and the CIHR Cell Signals Training programme. C.L. was supported by the CIHR Training Program in Regenerative Medicine (TPRM) and an Ontario graduate fellowship.
Author information
Authors and Affiliations
Contributions
C.X.L., B.H., N.P.T. and J.L.B. conceived the ideas and designed the experiments. C.X.L., E.K.-W., N.P.T., P.S., A.Koehler and S.B. performed experiments and analysed the data. C.X.L., B.H., N.P.T., A.Kapus and J.L.B. interpreted the data and wrote the manuscript. B.H. directed the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4736 kb)
Supplementary Information
Supplementary movie 1 (AVI 1683 kb)
Supplementary Information
Supplementary movie 2 (AVI 1293 kb)
Rights and permissions
About this article
Cite this article
Li, C., Talele, N., Boo, S. et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nature Mater 16, 379–389 (2017). https://doi.org/10.1038/nmat4780
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4780
This article is cited by
-
Fibroblast and myofibroblast activation in normal tissue repair and fibrosis
Nature Reviews Molecular Cell Biology (2024)
-
Linking cell mechanical memory and cancer metastasis
Nature Reviews Cancer (2024)
-
Cellular forgetting, desensitisation, stress and ageing in signalling networks. When do cells refuse to learn more?
Cellular and Molecular Life Sciences (2024)
-
Bone-matrix mineralization dampens integrin-mediated mechanosignalling and metastatic progression in breast cancer
Nature Biomedical Engineering (2023)
-
Mesenchymal stromal cells facilitate resolution of pulmonary fibrosis by miR-29c and miR-129 intercellular transfer
Experimental & Molecular Medicine (2023)