Abstract
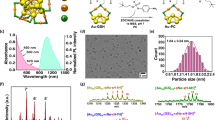
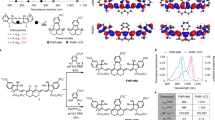
Fluorescent imaging of biological systems in the second near-infrared window (NIR-II) can probe tissue at centimetre depths and achieve micrometre-scale resolution at depths of millimetres. Unfortunately, all current NIR-II fluorophores are excreted slowly and are largely retained within the reticuloendothelial system, making clinical translation nearly impossible. Here, we report a rapidly excreted NIR-II fluorophore (∼90% excreted through the kidneys within 24 h) based on a synthetic 970-Da organic molecule (CH1055). The fluorophore outperformed indocyanine green (ICG)—a clinically approved NIR-I dye—in resolving mouse lymphatic vasculature and sentinel lymphatic mapping near a tumour. High levels of uptake of PEGylated-CH1055 dye were observed in brain tumours in mice, suggesting that the dye was detected at a depth of ∼4 mm. The CH1055 dye also allowed targeted molecular imaging of tumours in vivo when conjugated with anti-EGFR Affibody. Moreover, a superior tumour-to-background signal ratio allowed precise image-guided tumour-removal surgery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kamolz, L. P., Andel, H., Auer, T., Meissl, G. & Frey, M. Evaluation of skin perfusion by use of indocyanine green video angiography: Rational design and planning of trauma surgery. J. Trauma 61, 635–641 (2006).
Orth, D. H., Patz, A. & Flower, R. W. Potential clinical applications of indocyanine green choroidal angiography—preliminary-report. Eye Ear Nose Throat Mon. 55, 4–11 (1976).
Choi, H. S. et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nature Biotechnol. 31, 148–153 (2013).
Gioux, S., Choi, H. S. & Frangioni, J. V. Image-guided surgery using invisible near-infrared light: Fundamentals of clinical translation. Mol. Imaging 9, 237–255 (2010).
Hong, G. et al. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nature Med. 18, 1841–1846 (2012).
Liu, Z. et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nature Nanotech. 2, 47–52 (2007).
Vahrmeijer, A. L., Hutteman, M., van der Vorst, J. R., van de Velde, C. J. & Frangioni, J. V. Image-guided cancer surgery using near-infrared fluorescence. Nature Rev. Clin. Oncol. 10, 507–518 (2013).
Hong, G. et al. In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew. Chem. Int. Ed. 124, 9956–9959 (2012).
Hong, G. et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nature Commun. 5, 4206 (2014).
Diao, S. et al. Chirality enriched (12,1) and (11,3) single-walled carbon nanotubes for biological imaging. J. Am. Chem. Soc. 134, 16971–16974 (2012).
Choi, H. S. et al. Renal clearance of quantum dots. Nature Biotechnol. 25, 1165–1170 (2007).
Liu, Z. et al. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl Acad. Sci. USA 105, 1410–1415 (2008).
Antaris, A. L. et al. Ultra-low doses of chirality sorted (6,5) carbon nanotubes for simultaneous tumor imaging and photothermal therapy. ACS Nano 7, 3644–3652 (2013).
Welsher, K. et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature Nanotech. 4, 773–780 (2009).
Welsher, K., Sherlock, S. P. & Dai, H. J. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl Acad. Sci. USA 108, 8943–8948 (2011).
Fitzpatrick, J. A. J. et al. Long-term persistence and spectral blue shifting of quantum dots in vivo. Nano Lett. 9, 2736–2741 (2009).
Yang, S. T. et al. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol. Lett. 181, 182–189 (2008).
Xie, R., Chen, K., Chen, X. & Peng, X. InAs/InP/ZnSe core/shell/shell quantum dots as near-infrared emitters: Bright, narrow-band, non-cadmium containing, and biocompatible. Nano Res. 1, 457–464 (2008).
Hu, F. et al. Real-time in vivo visualization of tumor therapy by a near-infrared-II Ag2S quantum dot-based theranostic nanoplatform. Nano Res. 8, 1637–1647 (2015).
Tao, Z. M. et al. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew. Chem. Int. Ed. 52, 13002–13006 (2013).
Kim, S. et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature Biotechnol. 22, 93–97 (2004).
Maeda, H., Wu, J., Sawa, T., Matsumura, Y. & Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control Release 65, 271–284 (2000).
Wishart, G. C., Loh, S. W., Jones, L. & Benson, J. R. A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur. J. Surg. Oncol. 38, 651–656 (2012).
Gao, J. H. et al. Affibody-based nanoprobes for HER2-expressing cell and tumor imaging. Biomaterials 32, 2141–2148 (2011).
Qian, G. et al. Band gap tunable, donor–acceptor–donor charge-transfer heteroquinoid-based chromophores: Near infrared photoluminescence and electroluminescence. Chem. Mater. 20, 6208–6216 (2008).
Hong, G. et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nature Photon. 8, 723–730 (2014).
Murphy, J. E. et al. PbTe colloidal nanocrystals: Synthesis, characterization, and multiple exciton generation. J. Am. Chem. Soc. 128, 3241–3247 (2006).
Williams, A. T. R., Winfield, S. A. & Miller, J. N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 108, 1067–1071 (1983).
Ju, S. Y., Kopcha, W. P. & Papadimitrakopoulos, F. Brightly fluorescent single-walled carbon nanotubes via an oxygen-excluding surfactant organization. Science 323, 1319–1323 (2009).
Fox, M. E., Szoka, F. C. & Frechet, J. M. J. Soluble polymer carriers for the treatment of cancer: The importance of molecular architecture. Acc. Chem. Res. 42, 1141–1151 (2009).
Troyan, S. L. et al. The FLARE intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 16, 2943–2952 (2009).
Chi, C. W. et al. Use of indocyanine green for detecting the sentinel lymph node in breast cancer patients: From preclinical evaluation to clinical validation. PLoS ONE 8, e83927 (2013).
Tanaka, E., Choi, H. S., Fujii, H., Bawendi, M. G. & Frangioni, J. V. Image-guided oncologic surgery using invisible light: Completed pre-clinical development for sentinel lymph node mapping. Ann. Surg. Oncol. 13, 1671–1681 (2006).
Diao, S. et al. Biological imaging without autofluorescence in the second near-infrared window. Nano Res. 8, 3027–3034 (2015).
Zheng, M. B. et al. Robust ICG theranostic nanoparticles for folate targeted cancer imaging and highly effective photothermal therapy. ACS Appl. Mater. Interfaces 6, 6709–6716 (2014).
Gurfinkel, M. et al. Pharmacokinetics of ICG and HPPH-car for the detection of normal and tumor tissue using fluorescence, near-infrared reflectance imaging: A case study. Photochem. Photobiol. 72, 94–102 (2000).
Zimmermann, M., Zouhair, A., Azria, D. & Ozsahin, M. The epidermal growth factor receptor (EGFR) in head and neck cancer: Its role and treatment implications. Radiat. Oncol. 1, 11 (2006).
Miao, Z., Ren, G., Liu, H. G., Jiang, L. & Cheng, Z. Cy5.5-labeled Affibody molecule for near-infrared fluorescent optical imaging of epidermal growth factor receptor positive tumors. J. Biomed. Opt. 15, 36007 (2010).
Qi, S. B. et al. Evaluation of four affibody-based near-infrared fluorescent probes for optical imaging of epidermal growth factor receptor positive tumors. Bioconjug. Chem. 23, 1149–1156 (2012).
Ekblad, T. et al. Development and preclinical characterisation of Tc-99m-labelled Affibody molecules with reduced renal uptake. Eur. J. Nucl. Med. Mol. Imaging 35, 2245–2255 (2008).
Tolmachev, V. et al. Affibody molecules for epidermal growth factor receptor targeting in vivo: Aspects of dimerization and labeling chemistry. J. Nucl. Med. 50, 274–283 (2009).
Harris, J. M. & Chess, R. B. Effect of pegylation on pharmaceuticals. Nature Rev. Drug Discov. 2, 214–221 (2003).
He, X. X., Gao, J. H., Gambhir, S. S. & Cheng, Z. Near-infrared fluorescent nanoprobes for cancer molecular imaging: Status and challenges. Trends Mol. Med. 16, 574–583 (2010).
Acknowledgements
This work was partially supported by the Office of Science (BER), US Department of Energy (DE-SC0008397) (Z.C.), NCI Cancer Center Nanotechnology Excellence Grant (CCNE-TR) U54 CA119367, the Calbrain Program, a Neurotechnology Program of California (H.D.), CA151459, the National Natural Science Foundation of China 81573383, 81373254 and 21390402, NSFHP (2014CFB704) (X.Hong), International S&T Cooperation Program of China (2015DFA30440, 2014DFB30020) (X.Hong), the National Science and Technology Major Project of the Ministry of Science and Technology of China No. 2012ZX10004801-003-011 (X.Hong), Key Project of Chinese Ministry of Education No. 313040 (X.Hong), Academic Award for Excellent PhD Candidates funded by Ministry Of Education of China (no. 5052012306001), the Fundamental Research Funds for the Central Universities (C.Q., H.C., X.Hong) and Innovation Seed Fund of Wuhan University School of Medicine (X.Hong).
Author information
Authors and Affiliations
Contributions
H.D., Z.C. and X.Hong conceived and designed the study, supervised the project, and wrote the manuscript. A.L.A. and H.C. designed the study, performed all the experiments, and wrote the manuscript. C.Q., B.Z. and Y.S. contributed to synthesis of the compounds. K.C., X.Z., O.K.Y., G.H., S.D. and Z.R.A. helped with optical imaging. Z.D. and X.Hu contributed to the study design and preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 8200 kb)
Rights and permissions
About this article
Cite this article
Antaris, A., Chen, H., Cheng, K. et al. A small-molecule dye for NIR-II imaging. Nature Mater 15, 235–242 (2016). https://doi.org/10.1038/nmat4476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4476
This article is cited by
-
Image restoration of degraded time-lapse microscopy data mediated by near-infrared imaging
Nature Methods (2024)
-
Ratiometric fluorescent sensing of pyrophosphate with sp³-functionalized single-walled carbon nanotubes
Nature Communications (2024)
-
Oxyhaemoglobin saturation NIR-IIb imaging for assessing cancer metabolism and predicting the response to immunotherapy
Nature Nanotechnology (2024)
-
An ultra-small organic dye nanocluster for enhancing NIR-II imaging-guided surgery outcomes
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
NIR-IIb-triggered photodynamic therapy combined with chemotherapy platform based on rare-earth-doped nanoparticles
Rare Metals (2024)