Abstract
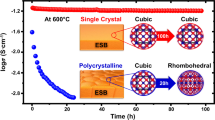
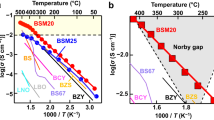
Bismuth-oxide-based materials are the building blocks for modern ferroelectrics1, multiferroics2, gas sensors3, light photocatalysts4 and fuel cells5,6. Although the cubic fluorite δ-phase of bismuth oxide (δ-Bi2O3) exhibits the highest conductivity of known solid-state oxygen ion conductors5, its instability prevents use at low temperature7,8,9,10. Here we demonstrate the possibility of stabilizing δ-Bi2O3 using highly coherent interfaces of alternating layers of Er2O3-stabilized δ-Bi2O3 and Gd2O3-doped CeO2. Remarkably, an exceptionally high chemical stability in reducing conditions and redox cycles at high temperature, usually unattainable for Bi2O3-based materials, is achieved. Even more interestingly, at low oxygen partial pressure the layered material shows anomalous high conductivity, equal or superior to pure δ-Bi2O3 in air. This suggests a strategy to design and stabilize new materials that are comprised of intrinsically unstable but high-performing component materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Park, B. H. et al. Lanthanum-substituted bismuth titanate for use in non-volatile memories. Nature 401, 682–684 (1999).
Wang, J. et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 299, 1719–1722 (2003).
Sears, W. M. The gas-sensing properties of sintered bismuth iron molybdate catalyst. Sensors Actuators 19, 351–370 (1989).
Gurunathan, K. Photocatalytic hydrogen production using transition metal ions-doped-Bi2O3 semiconductor particles. Int. J. Hydrog. Energy 29, 933–940 (2003).
Wachsman, E. D. & Lee, K. T. Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011).
Azad, A. M., Larose, S. & Akbar, S. A. Bismuth oxide-based solid electrolytes for fuel cells. J. Mater. Sci. 29, 4135–4151 (1994).
Kharton, V. V., Marques, F. M. B. & Atkinson, A. Transport properties of solid oxide electrolyte ceramics: A brief review. Solid State Ion. 174, 135–149 (2004).
Shuk, P., Wiemh, H. D., Guth, U., Göpel, W. & Greenblatt, M. Oxide ion conducting solid electrolytes based on Bi2O3 . Solid State Ion. 89, 179–196 (1996).
Takahashi, T. & Iwahara, H. High oxide ion conduction in sintered oxides of the system Bi2O3-WO3 . J. Appl. Electrochem. 3, 65–72 (1973).
Sammes, N. M., Tompsett, G. A., Nafe, H. & Aldinger, F. Bismuth based oxide electrolytes-structure and ionic conductivity. J. Eur. Ceram. Soc. 19, 1801–1826 (1999).
Battle, P. D., Catlow, C. R. A., Heap, J. W. & Moroney, L. M. Structural and dynamical studies of δ-Bi2O3 oxide-ion conductors II. A structural comparison of (Bi2O3)1−x(M2O3)x for M = Y, Er and Yb. J. Solid State Chem. 67, 42–50 (1987).
Jiang, N. & Wachsman, E. D. Structural stability and conductivity of phase-stabilized cubic bismuth oxides. J. Am. Ceram. Soc. 82, 3057–3064 (1999).
Boivin, J. C. & Thomas, D. Crystal chemistry and electrical properties of bismuth-based mixed oxides. Solid State Ion. 5, 523–525 (1981).
Boyapati, S., Wachsman, E. D. & Chakoumakos, B. C. Neutron diffraction study of occupancy and positional order of oxygen ions in phase-stabilized cubic bismuth oxides. Solid State Ion. 138, 293–304 (2001).
Boyapati, S., Wachsman, E. D. & Jiang, N. Effect of oxygen sublattice ordering on interstitial transport mechanism and conductivity activation energies in phase-stabilized cubic bismuth oxide. Solid State Ion. 140, 149–160 (2001).
Switzer, J. A., Shumsky, M. G. & Bohannan, E. W. Electrodeposited ceramic single crystals. Science 284, 293–296 (1999).
Lunca Popa, P. et al. Highly oriented δ-Bi2O3 thin films stable at room temperature synthesized by reactive magnetron sputtering. J. Appl. Phys. 113, 046101 (2013).
Laurent, K., Wang, G. Y., Tusseau-Nenez, S. & Leprince-Wang, Y. Structure and conductivity studies of electrodeposited δ-Bi2O3 . Solid State Ion. 178, 1735–1739 (2008).
Wachsman, E. D., Ball, G. R., Jiang, N. & Stevenson, D. A. Structural and defect studies in solid oxide electrolytes. Solid State Ion. 52, 213–218 (1992).
Takahashi, T., Esaka, T. & Iwahara, H. Conduction in Bi2O3-based oxide ion conductor under low oxygen pressure. II. Determination of the partial electronic conductivity. J. Appl. Electrochem. 7, 303–308 (1977).
Takahashi, T., Iwahara, H. & Nagai, Y. High oxide ion conduction in sintered Bi2O3 containing SrO, CaO or La2O3 . J. Appl. Electrochem. 2, 97–104 (1972).
Park, J. Y., Yoon, H. & Wachsman, E. D. Fabrication and characterization of high-conductivity bilayered electrolytes for intermediate-temperature solid oxide fuel cells. J. Am. Ceram. Soc. 88, 2402–2408 (2005).
Maier, J. Nanoionics: Ion transport and electrochemical storage in confined systems. Nature Mater. 4, 805–815 (2005).
Steele, B. C. H. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ion. 129, 95–110 (2000).
Garcia-Barriocanal, J. et al. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 321, 676–680 (2008).
Sata, N., Eberl, K., Eberman, K. & Maier, J. Mesoscopic fast ion conduction in nanometre-scale planar heterostructures. Nature 408, 946–949 (2000).
Sanna, S. et al. Enhancement of ionic conductivity in Sm-doped ceria/yttria-stabilized zirconia heteroepitaxial structures. Small 6, 1863–1867 (2010).
Schweiger, S., Kubicek, M., Messerschmitt, F., Murer, C. & Rupp, J. L. M. A. Microdot multilayer oxide device: Let us tune the strain-ionic transport interaction. ACS Nano 8, 5032–5048 (2014).
Ni, D. W. et al. Densification of highly defective ceria by high temperatures controlled reoxidation. J. Electrochem. Soc. 161, F1–F7 (2014).
Verkerk, M. J., Van de Velde, G. M. H. & Burggraaf, A. J. Structure and ionic conductivity of Bi2O3 substituted with lanthanide oxides. J. Phys. Chem. Solids 43, 1129–1136 (1982).
Acknowledgements
The authors would like to thank C. R. Graves for valuable suggestions and discussions and for critically reading the manuscript. O. Balmes is also acknowledged for his valuable help during the experiments at beam line ID03 at the ESRF. We also appreciate the help of E. Abdellahi with the preparation of (S)TEM specimens. We gratefully acknowledge The Danish Council for Independent Research |Natural Sciences, for travel support in connection with the synchrotron experiments, through the DANSCATT grant. The A.P. Møller and Chastine Mc-Kinney Møller Foundation are gratefully acknowledged for their contribution towards the establishment of the Center for Electron Nanoscopy in the Technical University of Denmark.
Author information
Authors and Affiliations
Contributions
S.S., V.E., S.L. and N.P. designed this project. V.E. elaborated the concept and S.S. developed the heterostructure layered architectures. S.S. deposited the thin films by PLD. S.S. and J.W.A. performed structural characterization by XRD and synchrotron light experiments. S.S. and M.C. performed structural characterization by high-temperature XRD. W.Z., T.K. and S.B.S. performed STEM and HRTEM characterizations. S.S., V.E. and J.H. performed the electrical characterization. S.S., V.E. and N.P. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1510 kb)
Rights and permissions
About this article
Cite this article
Sanna, S., Esposito, V., Andreasen, J. et al. Enhancement of the chemical stability in confined δ-Bi2O3. Nature Mater 14, 500–504 (2015). https://doi.org/10.1038/nmat4266
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4266
This article is cited by
-
A study of the microstructure and thermo–electrical properties of Bi2O3 ceramics co–doped with rare earth oxides
Journal of Materials Science: Materials in Electronics (2023)
-
Physically driven enhancement of the stability of Bi2O3-based ionic conductors via grain boundary engineering
NPG Asia Materials (2022)
-
Machine learning for a sustainable energy future
Nature Reviews Materials (2022)
-
Formation of a Ti → TiO2-graded layer and its effect on the memristive properties of TiOx(/Ti/TiOx) structures
Journal of Materials Science: Materials in Electronics (2022)
-
Oxygen vacancies in nanostructured hetero-interfacial oxides: a review
Journal of Nanoparticle Research (2022)