Abstract
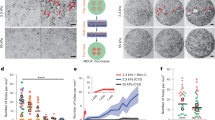
The origin of fracture in epithelial cell sheets subject to stretch is commonly attributed to excess tension in the cells’ cytoskeleton, in the plasma membrane, or in cell–cell contacts. Here, we demonstrate that for a variety of synthetic and physiological hydrogel substrates the formation of epithelial cracks is caused by tissue stretching independently of epithelial tension. We show that the origin of the cracks is hydraulic; they result from a transient pressure build-up in the substrate during stretch and compression manoeuvres. After pressure equilibration, cracks heal readily through actomyosin-dependent mechanisms. The observed phenomenology is captured by the theory of poroelasticity, which predicts the size and healing dynamics of epithelial cracks as a function of the stiffness, geometry and composition of the hydrogel substrate. Our findings demonstrate that epithelial integrity is determined in a tension-independent manner by the coupling between tissue stretching and matrix hydraulics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Alberts, B. Molecular Biology of the Cell (Garland Science, 2002).
Fung, Y. C. Biomechanics: Mechanical Properties of Living Tissues (Springer, 1993).
Guillot, C. & Lecuit, T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science 340, 1185–1189 (2013).
Bosveld, F. et al. Mechanical control of morphogenesis by fat/dachsous/four-jointed planar cell polarity pathway. Science 336, 724–727 (2012).
He, B., Doubrovinski, K., Polyakov, O. & Wieschaus, E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 508, 392–396 (2014).
Discher, D. et al. Biomechanics: Cell research and applications for the next decade. Ann. Biomed. Eng. 37, 847–859 (2009).
Fink, J. et al. External forces control mitotic spindle positioning. Nature Cell Biol. 13, 771–778 (2011).
Zhang, H. & Labouesse, M. Signalling through mechanical inputs: A coordinated process. J. Cell Sci. 125, 3039–3049 (2012).
Miller, C. J. & Davidson, L. A. The interplay between cell signalling and mechanics in developmental processes. Nature Rev. Genet. 14, 733–744 (2013).
The Acute Respiratory Distress Syndrome Network, Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Engl. J. Med. 342, 1301–1308 (2000).
Suki, B. & Hubmayr, R. Epithelial and endothelial damage induced by mechanical ventilation modes. Curr. Opin. Crit. Care 20, 17–24 (2014).
Harris, A. R. et al. Characterizing the mechanics of cultured cell monolayers. Proc. Natl Acad. Sci. USA 109, 16449–16454 (2012).
Trepat, X. et al. Viscoelasticity of human alveolar epithelial cells subjected to stretch. Am. J. Physiol. Lung Cell Mol. Physiol. 287, L1025–L1034 (2004).
Krishnan, R. et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am. J. Physiol. Cell Physiol. 300, C146–C154 (2011).
Dubrovskyi, O., Birukova, A. A. & Birukov, K. G. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab. Invest. 93, 254–263 (2013).
Sandre, O., Moreaux, L. & Brochard-Wyart, F. Dynamics of transient pores in stretched vesicles. Proc. Natl Acad. Sci. USA 96, 10591–10596 (1999).
Murrel, M. P. et al. Liposome adhesion generates traction stress. Nature Phys. 10, 163–169 (2014).
Vlahakis, N. E. & Hubmayr, R. D. Response of alveolar cells to mechanical stress. Curr. Opin. Crit. Care 9, 2–8 (2003).
Ostuni, E., Kane, R., Chen, C. S., Ingber, D. E. & Whitesides, G. M. Patterning mammalian cells using elastomeric membranes. Langmuir 16, 7811–7819 (2000).
Charras, G. T., Yarrow, J. C., Horton, M. A., Mahadevan, L. & Mitchison, T. J. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435, 365–369 (2005).
Paluch, E. K. & Raz, E. The role and regulation of blebs in cell migration. Curr. Opin. Cell Biol. 25, 582–590 (2013).
Maitre, J. L. et al. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338, 253–256 (2012).
Serra-Picamal, X. et al. Mechanical waves during tissue expansion. Nature Phys. 8, 628–634 (2012).
Trepat, X. et al. Physical forces during collective cell migration. Nature Phys. 5, 426–430 (2009).
Tambe, D. T. et al. Collective cell guidance by cooperative intercellular forces. Nature Mater. 10, 469–475 (2011).
Trepat, X. et al. Universal physical responses to stretch in the living cell. Nature 447, 592–595 (2007).
Gavara, N., Roca-Cusachs, P., Sunyer, R., Farre, R. & Navajas, D. Mapping cell-matrix stresses during stretch reveals inelastic reorganization of the cytoskeleton. Biophys. J. 95, 464–471 (2008).
Krishnan, R. et al. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS ONE 4, e5486 (2009).
Riveline, D. et al. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, 1175–1186 (2001).
Gardel, M. L. et al. Elastic behavior of cross-linked and bundled actin networks. Science 304, 1301–1305 (2004).
Roca-Cusachs, P., Iskratsch, T. & Sheetz, M. P. Finding the weakest link: Exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 125, 3025–3038 (2012).
Wolff, L., Fernández, P. & Kroy, K. Resolving the stiffening-softening paradox in cell mechanics. PLoS ONE 7, e40063 (2012).
Tanaka, T. & Fillmore, D. J. Kinetics of swelling of gels. J. Chem. Phys. 70, 1214–1218 (1979).
Noailly, J., Van Oosterwyck, H., Wilson, W., Quinn, T. M. & Ito, K. A poroviscoelastic description of fibrin gels. J. Biomech. 41, 3265–3269 (2008).
Moeendarbary, E. et al. The cytoplasm of living cells behaves as a poroelastic material. Nature Mater. 12, 253–261 (2013).
Hong, W., Zhao, X., Zhou, J. & Suo, Z. A theory of coupled diffusion and large deformation in polymeric gels. J. Mech. Phys. Solids 56, 1779–1793 (2008).
Li, J., Hu, Y., Vlassak, J. J. & Suo, Z. Experimental determination of equations of state for ideal elastomeric gels. Soft Matter 8, 8121–8128 (2012).
Derezic, D. & Cecuk, L. Hydrostatic pressure within renal cysts. Br. J. Urol. 54, 93–94 (1982).
West, J. B. & Mathieu-Costello, O. Vulnerability of pulmonary capillaries in heart disease. Circulation 92, 622–631 (1995).
Sonnemann, K. J. & Bement, W. M. Wound repair: Toward understanding and integration of single-cell and multicellular wound responses. Annu. Rev. Cell Dev. Biol. 27, 237–263 (2011).
Brugues, A. et al. Forces driving epithelial wound healing. Nature Phys. 10, 683–690 (2014).
Martinelli, R. et al. Release of cellular tension signals self-restorative ventral lamellipodia to heal barrier micro-wounds. J. Cell Biol. 201, 449–465 (2013).
Heo, J., Sachs, F., Wang, J. & Hua, S. Z. Shear-induced volume decrease in MDCK cells. Cell Physiol. Biochem. 30, 395–406 (2012).
Buehler, M. J. & Yung, Y. C. Deformation and failure of protein materials in physiologically extreme conditions and disease. Nature Mater. 8, 175–188 (2009).
Tang, Z., Kotov, N. A., Magonov, S. & Ozturk, B. Nanostructured artificial nacre. Nature Mater. 2, 413–418 (2003).
Espinosa, H. D. et al. Tablet-level origin of toughening in abalone shells and translation to synthetic composite materials. Nature Commun. 2, 173 (2011).
Gao, H., Ji, B., Jager, I. L., Arzt, E. & Fratzl, P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc. Natl Acad. Sci. USA 100, 5597–5600 (2003).
Jeffery, P. K. Remodeling in asthma and chronic obstructive lung disease. Am. J. Respir. Crit. Care Med. 164, S28–S38 (2001).
Coleman, H. R., Chan, C. C., Ferris, F. L. III & Chew, E. Y. Age-related macular degeneration. Lancet 372, 1835–1845 (2008).
Liu, F. et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 190, 693–706 (2010).
Simmons, C. S., Ribeiro, A. J. S. & Pruitt, B. L. Formation of composite polyacrylamide and silicone substrates for independent control of stiffness and strain. Lab Chip 13, 646–649 (2013).
Yeung, T. et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell. Motil. Cytoskeleton 60, 24–34 (2005).
Kandow, C. E., Georges, P. C., Janmey, P. A. & Beningo, K. A. Polyacrylamide hydrogels for cell mechanics: Steps toward optimization and alternative uses. Methods Cell Biol. 83, 29–46 (2007).
Nichols, J. E. et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng. A 19, 2045–2062 (2013).
Melo, E. et al. Effects of the decellularization method on the local stiffness of acellular lungs. Tissue Eng. C 20, 412–422 (2014).
Acknowledgements
We thank M. Bintanel, M. A. Rodríguez, J. Palou, E. Rebollo and N. Castro for technical assistance, the Nanotechnology platform at IBEC for microfabrication, P. Roca-Cusachs and members of the Trepat Lab for discussions, and B. Ladoux and J. De Rooij for plasmids and stable cell lines. This research was supported by the Spanish Ministry of Economy and Competitiveness (BFU2012-38146 to X.T., FIS-PI11/00089 to D.N.), the European Research Council (Grant Agreement 242993 to X.T., Grant Agreement 240487 to M.A.), and the National Institutes of Health (R01HL107561 to X.T.).
Author information
Authors and Affiliations
Contributions
L.C. and X.T. designed and implemented the experimental set-up. L.C., M.A. and X.T. designed the experiments, L.C. performed the experiments, and L.C. and R.V. analysed the experimental data. M.A. performed the theoretical analysis, D.Z. contributed technology, N.C. and D.N. decellularized the animal tissue, and L.C., M.A. and X.T. wrote the manuscript. All authors discussed and interpreted results, and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4226 kb)
Supplementary Information
Supplementary Movie 1 (MOV 609 kb)
Supplementary Information
Supplementary Movie 2 (MOV 314 kb)
Supplementary Information
Supplementary Movie 3 (MOV 752 kb)
Supplementary Information
Supplementary Movie 4 (MOV 385 kb)
Supplementary Information
Supplementary Movie 5 (MOV 308 kb)
Supplementary Information
Supplementary Movie 6 (MOV 510 kb)
Supplementary Information
Supplementary Movie 7 (MOV 435 kb)
Rights and permissions
About this article
Cite this article
Casares, L., Vincent, R., Zalvidea, D. et al. Hydraulic fracture during epithelial stretching. Nature Mater 14, 343–351 (2015). https://doi.org/10.1038/nmat4206
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4206
This article is cited by
-
Adherens junctions as molecular regulators of emergent tissue mechanics
Nature Reviews Molecular Cell Biology (2023)
-
Mechanical stress driven by rigidity sensing governs epithelial stability
Nature Physics (2023)
-
Patterning and dynamics of membrane adhesion under hydraulic stress
Nature Communications (2023)
-
How cells dig a hole for themselves
Nature Physics (2023)
-
Caveolin-1 dolines form a distinct and rapid caveolae-independent mechanoadaptation system
Nature Cell Biology (2023)