Abstract
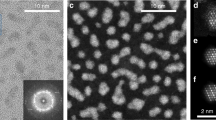
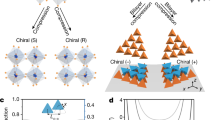
Self-assembled nanoparticle superlattices—materials made of inorganic cores capped by organic ligands, of varied structures, and held together by diverse binding motifs—exhibit size-dependent properties as well as tunable collective behaviour arising from couplings between their nanoscale constituents1,2,3,4,5,6,7,8,9,10,11,12,13,14,15. Here, we report the single-crystal X-ray structure of a superlattice made in the high-yield synthesis16 of Na4Ag44(p-MBA)30 nanoparticles, and find with large-scale quantum-mechanical simulations that its atomically precise structure and cohesion derive from hydrogen bonds between bundled4p-MBA ligands. We also find that the superlattice’s mechanical response to hydrostatic compression is characterized by a molecular-solid-like bulk modulus B0 = 16.7 GPa, exhibiting anomalous pressure softening and a compression-induced transition to a soft-solid phase. Such a transition involves ligand flexure, which causes gear-like correlated chiral rotation of the nanoparticles. The interplay of compositional diversity, spatial packing efficiency, hydrogen-bond connectivity, and cooperative response in this system exemplifies the melding of the seemingly contrasting paradigms of emergent behaviour ‘small is different’9 and ‘more is different’17.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
14 April 2014
In the version of this Letter originally published online, in Fig. 1a, the yellow arrow was duplicated. This error has now been corrected in all versions of the Letter.
References
Brus, L. Electronic wave-functions in semiconductor clusters-experiment and theory. J. Phys. Chem. 90, 2555–2560 (1986).
Murray, C. B., Kagan, C. R. & Bawendi, M. G. Self-organization of CdSe nanocrystals into three-dimensional quantum dot superlattices. Science 270, 1336–1338 (1995).
Whetten, R. L. et al. Nanocrystal gold molecules. Adv. Mater. 8, 428–433 (1996).
Luedtke, W. D. & Landman, U. Structure, dynamics, and thermodynamics of passivated gold nanocrystallites and their assemblies. J. Phys. Chem. 100, 13323–13329 (1996).
Bruchez, J. M., Moronne, M., Gin, P., Weiss, S. & Alivisatos, A. P. Semiconductor nanocrystals as fluorescent biological labels. Science 281, 2013–2016 (1998).
Whetten, R. L. et al. Crystal structures of molecular gold nanocrystal arrays. Acc. Chem. Res. 32, 397–406 (1999).
Link, S. & El-Sayed, M. A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 40, 8410–8426 (1999).
Maier, S. A. et al. Plasmonics - A route to nanoscale optical devices. Adv. Mater 13, 1501–1505 (2001).
Landman, U. & Luedtke, W.D. Small is different: Energetic, structural, thermal and mechanical properties of passivated nanocluster assemblies. Faraday Discuss. 125, 1–22 (2004).
Anker, J. N. et al. Biosensing with plasmonic nanosensors. Nature Mater. 7, 442–453 (2008).
Atwater, H. A. & Polman, A. Plasmonics for improved photovoltaic devices. Nature Mater. 9, 205–213 (2010).
Talapin, D. V., Lee, J-S., Kovalenko, M. V. & Shevchenko, E. V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 110, 389–458 (2010).
Goodfellow, B. W. & Korgel, B. A. Reversible solvent vapor-mediated phase changes in nanocrystal superlattices. ACS Nano 5, 2419–2424 (2011).
Hanrath, T. Colloidal nanocrystal quantum dot assemblies as artificial solids. J. Vac. Sci. Technol. A 30, 030802 (2012).
Arvizo, R. R. et al. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 41, 2943–2970 (2012).
Desireddy, A. et al. Ultrastable silver nanoparticles. Nature 501, 399–402 (2013).
Anderson, P. W. More is different. Science 177, 393–396 (1972).
Mueggenburg, K. E., Lin, X-M., Goldsmith, R. H. & Jaeger, H. M Elastic membranes of close-packed nanoparticle arrays. Nature Mater. 6, 656–660 (2007).
Podsiadlo, P. et al. The role of order, nanocrystal size, and capping ligands in the collective mechanical response of three-dimensional nanocrystal solids. J. Am. Chem. Soc. 132, 8953–8960 (2010).
Birch, F. Finite strain isotherm and velocities for single-crystal and polycrystalline NaCl at high pressures and 300° K. J. Geophys. Res. 83, 1257–1268 (1978).
Orgzall, I., Emmerling, F., Burkhard, S. B. & Franco, O. High-pressure studies on molecular crystals—relations between structure and high-pressure behavior. J. Phys. Condens. Matter 2, 1–15 (2008).
Syassena, K. & Holzapfel, W. B. Isothermal compression of AI and Ag to 120 kbar. J. Appl. Phys. 49, 4427–4430 (1978).
Yoshimura, Y., Stewart, S. T., Somayazulu, M., Mao, H-K. & Hemley, R. J. High-pressure X-ray diffraction and Raman spectroscopy of ice VIII. J. Chem. Phys. 124, 024502 (2006).
Collings, I. E. et al. Homologous critical behavior in the molecular frameworks Zn(CN)2 and Cd(imidazolate)2 . J. Am. Chem. Soc. 135, 7610–7620 (2013).
Luzar, A. & Chandler, D. Hydrogen-bond kinetics in liquid water. Nature 379, 55–57 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P. in Electronic Structure of Solids ’91 (eds Ziesche, P. & Eschrig, H.) Unified theory of exchange and correlation beyond the local density approximation. 11–20 (Akademie Verlag, 1991).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992); Erratum 48, 4978–4978 (1993)
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Desiraju, G. R. Supramolecular synthons in crystal engineering—a new organic synthesis. Angew. Chem. Int. Ed. 34, 2311–2327 (1995).
Tsiok, O. B., Brazhkin, V. V., Lyapin, A. G. & Khvostantsev, L. G. Logarithmic kinetics of the amorphous-amorphous transformations in SiO2 and GeO2 glasses under high-pressure. Phys. Rev. Lett. 80, 999–1002 (1998).
Pantea, C. et al. Pressure-induced elastic softening of monocrystalline zirconium tungstate at 300 K. Phys. Rev. B 73, 214118 (2006).
Khuong, T-A. V., Nuez, J. E., Godinez, C. E. & Garcia-Garibay, M. A. Crystalline molecular machines: A quest toward solid-state dynamics and function. Acc. Chem. Res. 39, 413–422 (2006).
Lakes, R. Deformation mechanisms in negative Poisson’s ratio materials: Structural aspects. J. Mater. Sci. 26, 2287–2292 (1991).
Acknowledgements
The work at GATECH by B.Y., R.N.B., W.D.L. and U.L. was supported by a grant from the Air Force Office of Scientific Research, and the work of J.G. was supported by the Office of Basic Energy Sciences of the US Department of Energy under Contract No. FG05-86ER45234. Computations were made at the Georgia Tech Center for Computational Materials Science. Work at the University of Toledo was supported by NSF grants CHE-1012896 and CRIF-0840474.
Author information
Authors and Affiliations
Contributions
U.L. conceived and directed the theoretical work, analysed the experimental and theoretical results, and wrote the manuscript. B.Y. performed the DFT computations. B.Y., W.D.L., R.N.B. and J.G. participated in the analysis of the computational and experimental results. T.P.B. conceived, directed and analysed the experimental research, and A.D. and B.E.C. performed the experimental work. All authors contributed to preparation of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2970 kb)
Rights and permissions
About this article
Cite this article
Yoon, B., Luedtke, W., Barnett, R. et al. Hydrogen-bonded structure and mechanical chiral response of a silver nanoparticle superlattice. Nature Mater 13, 807–811 (2014). https://doi.org/10.1038/nmat3923
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3923
This article is cited by
-
Tuning three-dimensional nano-assembly in the mesoscale via bis(imino)pyridine molecular functionalization
Scientific Reports (2022)
-
Assembly-induced spin transfer and distance-dependent spin coupling in atomically precise AgCu nanoclusters
Nature Communications (2022)
-
Superatoms in materials science
Nature Reviews Materials (2020)
-
Control of single-ligand chemistry on thiolated Au25 nanoclusters
Nature Communications (2020)
-
Directed assembly of magnetic and semiconducting nanoparticles with tunable and synergistic functionality
Scientific Reports (2019)