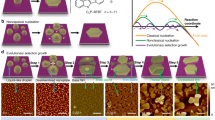
Abstract
Additives, including nucleating agents, have been used to regulate the solidification process of (semi-)crystalline polymer solids and thus control both their crystallite dimensions and shape1,2,3,4,5. Here, we demonstrate that minute amounts (0.1–1 wt%) of commercially available nucleating agents can be used to efficiently manipulate the solidification kinetics of a wide range of organic semiconductors—including poly(3-alkylthiophene)s, the fullerene derivative [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) and 6,13-bis(triisopropyl-silylethynyl) (TIPS) pentacene—when processed from the melt, solution or solid state, without adversely affecting the semiconductors’ electronic properties. Heterogeneous nucleation increases the temperature of and rate of crystallization of poly(3-alkylthiophene)s, permits patterning of crystallites at pre-defined locations in PCBM, and minimizes dewetting of films of TIPS-pentacene formed by inkjet printing. Nucleating agents thus make possible the fabrication of thin-film transistors with uniform electrical characteristics at high yield.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kristiansen, M. et al. The binary system isotactic polypropylene/bis(3,4-dimethylbenzylidene)sorbitol: Phase behavior, nucleation, and optical properties. Macromolecules 36, 5150–5156 (2003).
Kristiansen, M., Tervoort, T., Smith, P. & Goossens, H. Mechanical properties of sorbitol-clarified isotactic polypropylene: Influence of additive concentration on polymer structure and yield behavior. Macromolecules 38, 10461–10465 (2005).
Kristiansen, P. M., Gress, A., Smith, P., Hanft, D. & Schmidt, H. W. Phase behavior, nucleation and optical properties of the binary system isotactic polypropylene/N, N′, N-tris-isopentyl-1,3,5-benzene-tricarboxamide. Polymer 47, 249–253 (2006).
Kristiansen, M. et al. Structural aspects of 1,3,5-benzenetrisamides-a new family of nucleating agents. Cryst. Growth Design 9, 2556–2558 (2009).
Blomenhofer, M. et al. ‘Designer’ nucleating agents for polypropylene. Macromolecules 38, 3688–3695 (2005).
Giri, G. et al. Tuning charge transport in solution-sheared organic semiconductors using lattice strain. Nature 480, 504–508 (2011).
Yan, H. et al. A high-mobility electron-transporting polymer for printed transistors. Nature 457, 679–686 (2009).
Bronstein, H. et al. Thieno 3,2-b thiophene-diketopyrrolopyrrole-containing polymers for high-performance organic field-effect transistors and organic photovoltaic devices. J. Am. Chem. Soc. 133, 3272–3275 (2011).
Liang, Y. Y. et al. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 22, E135–E138 (2010).
Peet, J. et al. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nature Mater. 6, 497–500 (2007).
Sun, Y et al. Solution-processed small-molecule solar cells with 6.7% efficiency. Nature Mater. 11, 44–48 (2012).
Moon, J. S. et al. Effect of processing additive on the nanomorphology of a bulk heterojunction material. Nano Lett. 10, 4005–4008 (2010).
Rogers, J. T., Schmidt, K., Toney, M. F., Bazan, G. C. & Kramer, E. J. Time-resolved structural evolution of additive-processed bulk heterojunction solar cells. J. Am. Chem. Soc. 134, 2884–2887 (2012).
Lee, J. K. et al. Processing additives for improved efficiency from bulk heterojunction solar cells. J. Am. Chem. Soc. 130, 3619–3623 (2008).
Chen, F-C., Tseng, H-C. & Ko, C-J. Solvent mixtures for improving device fficiency of polymer photovoltaic devices. Appl. Phys. Lett. 92, 103316 (2008).
Stingelin-Stutzmann, N. et al. Organic thin-film electronics from vitreous solution-processed rubrene hypereutectics. Nature Mater. 4, 601–606 (2005).
Lee, S. S. et al. Understanding heterogeneous nucleation in binary, solution-processed, organic semiconductor thin films. Chem. Mater. 24, 2920–2928 (2012).
Krache, R. et al. Competition between α, β, and γ polymorphs in a β-nucleated metallocenic isotactic polypropylene. Macromolecules 40, 6871–6878 (2007).
Wunderlich, B. Macromolecular Physics: Crystal Nucleation, Growth, Annealing (Academic, 1976).
Gornick, F. & Hoffman, J. D. Nucleation in polymers. Ind. Eng. Chem. 58, 41–53 (1966).
Reid, O. G. et al. The influence of solid-state microstructure on the origin and yield of long-lived photogenerated charge in neat semiconducting polymers. J. Polym. Sci. B 50, 27–37 (2012).
Paquin, F. et al. Charge separation in semicrystalline polymeric semiconductors by photoexcitation: Is the mechanism intrinsic or extrinsic?. Phys. Rev. Lett. 106, 197401 (2011).
Arias, A. C., MacKenzie, J. D., McCulloch, I., Rivnay, J. & Salleo, A. Materials and applications for large area electronics: Solution-based approaches. Chem. Rev. 110, 3–24 (2010).
Virkar, A. A., Mannsfeld, S., Bao, Z. & Stingelin, N. Organic semiconductor growth and morphology considerations for organic thin-film transistors. Adv. Mater. 22, 3857–3875 (2010).
Krebs, F. C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Ener. Mater. Sol. Cells 93, 394–412 (2009).
Choulis, S. A. et al. High ambipolar and balanced carrier mobility in regioregular poly(3-hexylthiophene). Appl. Phys. Lett. 85, 3890–3892 (2004).
Tanase, C., Meijer, E. J., Blom, P. W. M. & de Leeuw, D. M. Unification of the hole transport in polymeric field-effect transistors and light-emitting diodes. Phys. Rev. Lett. 91, 216601 (2003).
Tuladhar, S. M. et al. Ambipolar charge transport in films of methanofullerene and poly(phenylenevinylene)/methanofullerene blends. Adv. Funct. Mater. 15, 1171–1182 (2005).
Spano, F. C., Clark, J., Silva, C. & Friend, R. H. Determining exciton coherence from the photoluminescence spectral line shape in poly(3-hexylthiophene) thin films. J. Chem. Phys. 130, 074904–074916 (2009).
Park, S. K., Jackson, T. N., Anthony, J. E. & Mourey, D. A. High mobility solution processed 6,13-bis(triisopropyl-silylethynyl) pentacene organic thin film transistors. Appl. Phys. Lett. 91, 063514 (2007).
Treat, N. D. et al. Polymer-fullerene miscibility: A metric for screening new materials for high-performance organic solar cells. J. Am. Chem. Soc. 134, 15869–15879 (2012).
Collins, B. A. et al. Absolute measurement of domain composition and nanoscale size distribution explains performance in PTB7:PC71BM solar cells. Adv. Ener. Mater. 3, 65–74 (2013).
Binsbergen, F. L. Heterogeneous nucleation in the crystallization of polyolefins. III. Theory and mechanism. J. Polym. Science 11, 117–135 (1973).
Acknowledgements
N.D.T. acknowledges support from the NSF IRFP (OISE-1201915), NSF ConvEne IGERT Program (NSF-DGE 0801627), and NSF Graduate Research Fellowship. C.G.S., C.J.H. and M.L.C. thank the NSF ICC program (CHE-1026664) and the NSF SOLAR program (CHE-1035292) for additional support of this work. Portions of this research (N.D.T., C.J.H. and M.L.C.) were carried out at the MRL Central Facilities, which are supported by the MRSEC Program of the NSF under Award No. DMR-1121053; a member of the NSF-funded Materials Research Facilities Network (www.mrfn.org). C.G.S. was supported as part of the Center for Energy Efficient Materials, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001009. G.R. and O.R. acknowledge Laboratory Directed Research and Development (LDRD) funding under award number 06RF1201 from NREL. We are also very grateful to the UK’s Engineering and Physical Sciences Research Council (EP/G060738/1), the Dutch Polymer Institute (LATFE programme) and the ACS Petroleum Fund (New Directions Proposal) for financial support. N.S. is furthermore supported by a European Research Council (ERC) Starting Independent Researcher Fellowship under the grant agreement No. 279587. Portions of this research were also carried out at the SSRL, a national user facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences.
Author information
Authors and Affiliations
Contributions
N.D.T. prepared and characterized samples, except for those used in OFETs. L.Y. prepared and characterized the OFETs. J.A.N.M. prepared samples for DSC and TRMC, C.G.S. performed TOF measurements, and O.R. prepared TRMC samples and performed TRMC measurements. N.D.T., N.S., M.L.C., C.J.H, P.S., and G.R. designed the experiments and prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1333 kb)
Rights and permissions
About this article
Cite this article
Treat, N., Nekuda Malik, J., Reid, O. et al. Microstructure formation in molecular and polymer semiconductors assisted by nucleation agents. Nature Mater 12, 628–633 (2013). https://doi.org/10.1038/nmat3655
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3655
This article is cited by
-
Matrix Effect on Polydiarylfluorenes Electrospun Hybrid Microfibers: From Morphology Tuning to High Explosive Detection Efficiency
Chinese Journal of Polymer Science (2023)
-
Small-molecule additives for organic thin film transistors
Journal of Materials Science: Materials in Electronics (2019)
-
Crystallization and Polymorphism of Organic Semiconductor in Thin Film Induced by Surface Segregated Monolayers
Scientific Reports (2018)
-
The meniscus-guided deposition of semiconducting polymers
Nature Communications (2018)
-
Conjugation break spacers and flexible linkers as tools to engineer the properties of semiconducting polymers
Polymer Journal (2018)