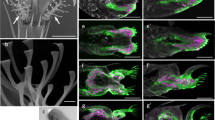
Abstract
A promising approach to improve the performance of microelectronic devices is to build three-dimensional (3D) chips made of stacked circuits. However, a major hurdle lies in the fabrication of dense arrays of electrical interconnections between these layers, where accessibility is limited1,2. Here we show that the directed growth and self-organization of actin filaments can offer a solution to this problem. We defined the shape and orientation of 3D actin networks through both micropatterning of actin nucleation factors and biochemical control of actin filament polymerization. Networks growing from two opposing layers were able to interpenetrate and form mechanically stable connections, which were then coated with gold using a selective metallization process. The electrical conductivity, robustness and modularity of the metallized self-organized connections make this approach potentially attractive for 3D chip manufacturing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Farooq, M. G. & Iyer, S. S. 3D integration review. Sci. China Inform. Sci. 54, 1012–1025 (2011).
Garrou, P., Bower, C. & Ramm, P. Handbook of 3D Integration: Technology and Applications of 3D Integrated Circuits (Wiley, 2008).
Emma, P. G. & Kursun, E. Is 3D chip technology the next growth engine for performance improvement? IBM J. Res. Dev. 52, 541–552 (2008).
Cale, T. S., Lu, J-Q. & Gutmann, R. J. Three-dimensional integration in microelectronics: Motivation, processing, and thermo mechanical modeling. Chem. Eng. Commun. 195, 847–888 (2008).
Leduc, P. et al. 2009 IEEE Int. Conf. on 3D System Integration 1–5 (IEEE, 2009).
Jourdain, A. et al. IEEE 61st Electronic Components and Technology Conf. 1122–1125 (IEEE, 2011).
Winfree, E., Liu, F., Wenzler, L. A. & Seeman, N. C. Design and self-assembly of two-dimensional DNA crystals. Nature 394, 539–544 (1998).
Rothemund, P. W. K. Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302 (2006).
Douglas, S. M. et al. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459, 414–418 (2009).
Han, D. et al. DNA origami with complex curvatures in three-dimensional space. Science 332, 342–346 (2011).
Dinca, V. et al. Directed three-dimensional patterning of self-assembled peptide fibrils. Nano Lett. 8, 538–543 (2008).
Adler-Abramovich, L. et al. Thermal and chemical stability of diphenylalanine peptide nanotubes: Implications for nanotechnological applications. Langmuir 22, 1313–1320 (2006).
Huang, Y. et al. Programmable assembly of nanoarchitectures using genetically engineered viruses. Nano Lett. 5, 1429–1434 (2005).
Scanlon, S. & Aggeli, A. Self-assembling peptide nanotubes. Nano Today 3, 22–30 (June–August, 2008).
Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nature Biotechnol. 21, 1171–1178 (2003).
Portran, D., Gaillard, J., Vantard, M. & Thery, M. Quantification of MAP and molecular motor activities on geometrically controlled microtubule networks. Cytoskeleton 70, 12–23 (2013).
Reymann, A-C. et al. Nucleation geometry governs ordered actin networks structures. Nature Mater. 9, 827–832 (2010).
Gazit, E. Use of biomolecular templates for the fabrication of metal nanowires. FEBS J. 274, 317–322 (2007).
Patolsky, F., Weizmann, Y. & Willner, I. Actin-based metallic nanowires as bio-nanotransporters. Nature Mater. 3, 692–695 (2004).
Zhou, J. C. et al. Microtubule-based gold nanowires and nanowire arrays. Small 4, 1507–1515 (2008).
Braun, E., Eichen, Y., Sivan, U. & Ben-Yoseph, G. DNA-templated assembly and electrode attachment of a conducting silver wire. Nature 391, 775–778 (1998).
Pearson, A. C. et al. DNA origami metallized site specifically to form electrically conductive nanowires. J. Phys. Chem. B 116, 10551–10560 (2012).
Michelot, A. & Drubin, D. G. Building distinct actin filament networks in a common cytoplasm. Curr. Biol. 21, R560–R569 (2011).
Blanchoin, L. et al. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 171, 1007–1011 (2000).
Loisel, T. P., Boujemaa, R., Pantaloni, D. & Carlier, M. F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 613–616 (1999).
Oudenaarden, A. Van & Theriot, J. A. Cooperative symmetry-breaking by actin polymerization in a model for cell motility. Nature Cell Biol. 493–499 (1999).
Van der Gucht, J., Paluch, E., Plastino, J. & Sykes, C. Stress release drives symmetry breaking for actin-based movement. Proc. Natl Acad. Sci. USA 102, 7847–7852 (2005).
Achard, V. et al. A ‘primer’-based mechanism underlies branched actin filament network formation and motility. Curr. Biol. 20, 423–428 (2010).
Vignaud, T. et al. Reprogramming cell shape with laser nano-patterning. J. Cell Sci. 125, 2134–2140 (2012).
Behrens, S., Habicht, W., Wagner, K. & Unger, E. Assembly of nanoparticle ring structures based on protein templates. Adv. Mater. 18, 284–289 (2006).
Scheibel, T. et al. Conducting nanowires built by controlled self-assembly of amyloid fibres and selective metal deposition. Proc. Natl Acad. Sci. USA 100, 4527–4532 (2003).
Azioune, A., Carpi, N., Tseng, Q., Théry, M. & Piel, M. Protein micropatterns: A direct printing protocol using deep UVs. Methods Cell Biol. 97, 133–146 (2010).
Acknowledgements
This work was supported by the ‘Chimtronique’ programme of the CEA. It has been performed with the help of the ‘Plateforme technologique amont’ of Grenoble, and with the financial support of the ‘Nanosciences aux limites de la Nanoélectronique’ Foundation. We thank J-C. Gabriel for constructive discussions, F. Perraut for technological advice and C. Suarez for his support in biochemistry.
Author information
Authors and Affiliations
Contributions
R.G., C.G. and P.L. carried out the experiments. D.P., L.B. and M.T. directed the project. R.G., L.B. and M.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The CEA and the CNRS have filed an application for a patent on the technology described in this manuscript, of which R.G., L.B. and M.T. are inventors.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1159 kb)
Rights and permissions
About this article
Cite this article
Galland, R., Leduc, P., Guérin, C. et al. Fabrication of three-dimensional electrical connections by means of directed actin self-organization. Nature Mater 12, 416–421 (2013). https://doi.org/10.1038/nmat3569
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3569
This article is cited by
-
The discovery of actin: “to see what everyone else has seen, and to think what nobody has thought”*
Journal of Muscle Research and Cell Motility (2020)
-
Disordered protein-graphene oxide co-assembly and supramolecular biofabrication of functional fluidic devices
Nature Communications (2020)
-
Directing curli polymerization with DNA origami nucleators
Nature Communications (2019)
-
Costameres, dense plaques and podosomes: the cell matrix adhesions in cardiovascular mechanosensing
Journal of Muscle Research and Cell Motility (2019)
-
Efficient DNA-assisted synthesis of trans-membrane gold nanowires
Microsystems & Nanoengineering (2018)