Abstract
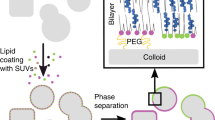
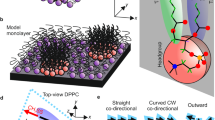
Liquid-crystalline phases of stacked lipid bilayers represent a pervasive motif in biomolecular assemblies. Here we report that, in addition to the usual smectic order, multicomponent multilayer membranes can exhibit columnar order arising from the coupling of two-dimensional intralayer phase separation and interlayer smectic ordering. This coupling propagates across hundreds of membrane lamellae, producing long-range alignment of phase-separated domains. Quantitative analysis of real-time dynamical experiments reveals that there is an interplay between intralayer domain growth and interlayer coupling, suggesting the existence of cooperative multilayer epitaxy. We postulate that such long-range epitaxy is solvent-assisted, and that it originates from the surface tension associated with differences in the network of hydrogen-bonded water molecules at the hydrated interfaces between the domains and the surrounding phase. Our findings might inspire the development of self-assembly-based strategies for the long-range alignment of functional lipid domains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Levine, Y. K., Bailey, A. I. & Wilkins, M. H. F. Multilayers of phospholipid bimolecular leaflets. Nature 220, 577–578 (1968).
Schmitz, G. & Muller, G. Structure and function of lamellar bodies, lipid–protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 32, 1539–1570 (1991).
Bald, D., Kruip, J. & Rogner, M. Supramolecular architecture of cyanobacterial thylakoid membranes: How is the phycobilisome connected with the photosystems? Photosynth. Res. 49, 103–118 (1996).
Keegstra, K. & Cline, K. Protein import and routing systems of chloroplasts. Plant Cell 11, 557–570 (1999).
Powers, L. & Clark, N. A. Preparation of large monodomain phospholipid bilayer smectic liquid-crystals. Proc. Natl Acad. Sci USA 72, 840–843 (1975).
Xu, J. & Lavan, D. A. Designing artificial cells to harness the biological ion concentration gradient. Nature Nanotech. 3, 666–670 (2008).
Lenhert, S. et al. Lipid multilayer gratings. Nature Nanotech. 5, 275–279 (2010).
Radler, J. O., Koltover, I., Salditt, T. & Safinya, C. R. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science 275, 810–814 (1997).
Yamamoto, J. & Tanaka, H. Dynamic control of the photonic smectic order of membranes. Nature Mater. 4, 75–80 (2005).
Almeida, P. F. F. Thermodynamics of lipid interactions in complex bilayers. Biochim. Biophys. Acta-Biomembranes 1788, 72–85 (2009).
Brown, D. A. & London, E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14, 111–136 (1998).
Simons, K. & Ikonen, E. Cell biology—How cells handle cholesterol. Science 290, 1721–1726 (2000).
Chazal, N. & Gerlier, D. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67, 226–237 (2003).
Martens, J. R. et al. Differential targeting of shaker-like potassium channels to lipid rafts. J. Biol. Chem. 275, 7443–7446 (2000).
Yarbrough, T. L., Lu, T., Lee, H. C. & Shibata, E. F. Localization of cardiac sodium channels in caveolin-rich membrane domains—regulation of sodium current amplitude. Circ. Res. 90, 443–449 (2002).
Darby, P. J., Kwan, C. Y. & Daniel, E. E. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca2+ handling. Am. J. Physiol-Lung Cell. Mol. Physiol. 279, L1226–L1235 (2000).
Seul, M. & Sammon, M. J. Preparation of surfactant multilayer films on solid substrates by deposition from organic solution. Thin Solid Films 185, 287–305 (1990).
Du, X. Y., Whallon, J. H. & Hollingsworth, R. I. Characterization of lipid multilayer microphase structures by phase-contrast and confocal-reflection microscopies. Langmuir 14, 5581–5585 (1998).
Dietrich, C. et al. Lipid rafts reconstituted in model membranes. Biophys. J. 80, 1417–1428 (2001).
Samsonov, A. V., Mihalyov, I. & Cohen, F. S. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys. J. 81, 1486–1500 (2001).
Veatch, S. L. & Keller, S. L. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 89, 268101 (2002).
Veatch, S. L. & Keller, S. L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85, 3074–3083 (2003).
Baumgart, T., Hess, S. T. & Webb, W. W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 425, 821–824 (2003).
Baumgart, T., Hunt, G., Farkas, E. R., Webb, W. W. & Feigenson, G. W. Fluorescence probe partitioning between Lo/Ld phases in lipid membranes. Biochim. Biophys. Acta-Biomembranes 1768, 2182–2194 (2007).
Veatch, S. L. & Keller, S. L. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 94, 148101 (2005).
Ziblat, R., Leiserowitz, L. & Addadi, L. Crystalline domain structure and cholesterol crystal nucleation in single hydrated DPPC: cholesterol:POPC bilayers. J. Am. Chem. Soc. 132, 9920–9927 (2010).
Lifshitz, I. M. & Slyozov, V. V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 19, 35–50 (1961).
Jensen, M. H., Morris, E. J. & Simonsen, A. C. Domain shapes, coarsening, and random patterns in ternary membranes. Langmuir 23, 8135–8141 (2007).
Rogers, T. M. & Desai, R. C. Numerical study of late-stage coarsening for off-critical quenches in the Cahn–Hilliard equation of phase-separation. Phys. Rev. B 39, 11956–11964 (1989).
Rinia, H. A., Snel, M. M. E., van der Eerden, J. & de Kruijff, B. Visualizing detergent resistant domains in model membranes with atomic force microscopy. FEBS Lett. 501, 92–96 (2001).
Van Duyl, B. Y., Ganchev, D., Chupin, V., de Kruijff, B. & Killian, J. A. Sphingomyelin is much more effective than saturated phosphatidylcholine in excluding unsaturated phosphatidylcholine from domains formed with cholesterol. FEBS Lett. 547, 101–106 (2003).
Tristram-Nagle, S. & Nagle, J. F. Lipid bilayers: thermodynamics, structure, fluctuations, and interactions. Chem. Phys. Lipids 127, 3–14 (2004).
Garcia-Saez, A. J., Chiantia, S. & Schwille, P. Effect of line tension on the lateral organization of lipid membranes. J. Biol. Chem. 282, 33537–33544 (2007).
Kuzmin, P. I., Akimov, S. A., Chizmadzhev, Y. A., Zimmerberg, J. & Cohen, F. S. Line tension and interaction energies of membrane rafts calculated from lipid splay and tilt. Biophys. J. 88, 1120–1133 (2005).
Cheng, J. X., Pautot, S., Weitz, D. A. & Xie, X. S. Ordering of water molecules between phospholipid bilayers visualized by coherent anti-Stokes Raman scattering microscopy. Proc. Natl Acad. Sci USA 100, 9826–9830 (2003).
Milhaud, J. New insights into water-phospholipid model membrane interactions. Biochim. Biophys. Acta-Biomembranes 1663, 19–51 (2004).
Marrink, S. J., Berkowitz, M. & Berendsen, H. J. C. Molecular-dynamics simulation of a membrane water interface—the ordering of water and its relation to the hydration force. Langmuir 9, 3122–3131 (1993).
Leikin, S., Parsegian, V. A., Rau, D. C. & Rand, R. P. Hydration forces. Annu. Rev. Phys. Chem. 44, 369–395 (1993).
Marcelja, S. & Radic, N. Repulsion of interfaces due to boundary water. Chem. Phys. Lett. 42, 129–130 (1976).
Attard, P. & Batchelor, M. T. A mechanism for the hydration force demonstrated in a model system. Chem. Phys. Lett. 149, 206–211 (1988).
Kornyshev, A. A. & Leikin, S. Fluctuation theory of hydration forces—the dramatic effects of inhomogeneous boundary-conditions. Phys. Rev. A 40, 6431–6437 (1989).
Rand, R. P., Das, S. & Parsegian, V. A. The hydration force, its character, universality and application—some current issues. Chem. Scr. 25, 15–21 (1985).
Jendrasiak, G. L. & Smith, R. L. The effect of the choline head group on phospholipid hydration. Chem. Phys. Lipids 113, 55–66 (2001).
Fernandez, A. Epistructural tension promotes protein associations. Phys. Rev. Lett. 108, 188102 (2012).
Fenimore, P. W., Frauenfelder, H., McMahon, B. H. & Young, R. D. Bulk-solvent and hydration-shell fluctuations, similar to alpha- and beta-fluctuations in glasses, control protein motions and functions. Proc. Natl Acad. Sci USA 101, 14408–14413 (2004).
Salditt, T., Li, C., Spaar, A. & Mennicke, U. X-ray reflectivity of solid-supported, multilamellar membranes. Eur. Phys. J. E 7, 105–116 (2002).
Wiener, M. C. & White, S. H. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of X-ray and neutron-diffraction data. III. Complete structure. Biophys. J. 61, 434–447 (1992).
Acknowledgements
This work is supported by a grant from the Biomolecular Materials Program, Division of Materials Science and Engineering, Basic Energy Sciences, US Department of Energy under Award no. DE-FG02-04ER46173.
Author information
Authors and Affiliations
Contributions
L.T., Y. M. and G.C. performed experiments, analysed data and wrote initial drafts of the manuscript. D.V. contributed extensively to all experimental data analyses and helped L.T. with the theoretical model. A.N.P. and S.K.S. conceived the overall project, provided intellectual and technical guidance, performed final edits of the manuscript and are principal investigators of the supporting grant. All authors contributed to writing and revising the manuscript, and agreed on its final content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 520 kb)
Supplementary Information
Supplementary Movie S1 (AVI 476 kb)
Supplementary Information
Supplementary Movie S2 (AVI 3327 kb)
Supplementary Information
Supplementary Movie S3 (AVI 933 kb)
Rights and permissions
About this article
Cite this article
Tayebi, L., Ma, Y., Vashaee, D. et al. Long-range interlayer alignment of intralayer domains in stacked lipid bilayers. Nature Mater 11, 1074–1080 (2012). https://doi.org/10.1038/nmat3451
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3451
This article is cited by
-
Real-time cholesterol sorting in Plasmodium falciparum-erythrocytes as revealed by 3D label-free imaging
Scientific Reports (2020)
-
Complex biomembrane mimetics on the sub-nanometer scale
Biophysical Reviews (2017)
-
Line tension at lipid phase boundaries as driving force for HIV fusion peptide-mediated fusion
Nature Communications (2016)
-
HIV gp41–mediated membrane fusion occurs at edges of cholesterol-rich lipid domains
Nature Chemical Biology (2015)
-
Controls and constrains of the membrane disrupting action of Aurein 1.2
Scientific Reports (2015)