Abstract
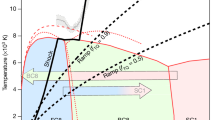
Graphite and diamond have comparable free energies, yet forming diamond from graphite in the absence of a catalyst requires pressures that are significantly higher than those at equilibrium coexistence1,2,3,4,5,6,7. At lower temperatures, the formation of the metastable hexagonal polymorph of diamond is favoured instead of the more stable cubic diamond2,5,6,7. These phenomena cannot be explained by the concerted mechanism suggested in previous theoretical studies8,9,10,11,12. Using an ab initio quality neural-network potential13, we carried out a large-scale study of the graphite-to-diamond transition assuming that it occurs through nucleation. The nucleation mechanism accounts for the observed phenomenology and reveals its microscopic origins. We demonstrate that the large lattice distortions that accompany the formation of diamond nuclei inhibit the phase transition at low pressure, and direct it towards the hexagonal diamond phase at higher pressure. The proposed nucleation mechanism should improve our understanding of structural transformations in a wide range of carbon-based materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bundy, F. P. Direct conversion of graphite to diamond in static pressure apparatus. J. Chem. Phys. 38, 631–643 (1963).
Bundy, F. P. & Kasper, J. S. Hexagonal diamond—a new form of carbon. J. Chem. Phys. 46, 3437–3446 (1967).
Bundy, F. P. et al. The pressure–temperature phase and transformation diagram for carbon; updated through 1994. Carbon 34, 141–153 (1996).
Irifune, T., Kurio, A., Sakamoto, S., Inoue, T. & Sumiya, H. Ultrahard polycrystalline diamond from graphite. Nature 421, 599–600 (2003).
Britun, V. F., Kurdyumov, A. V. & Petrusha, I. A. Diffusionless nucleation of lonsdaleite and diamond in hexagonal graphite under static compression. Powder Metall. Met. Ceram. 43, 87–93 (2004).
Sumiya, H., Yusa, H., Inoue, T., Ofuji, H. & Irifune, T. Conditions and mechanism of formation of nano-polycrystalline diamonds on direct transformation from graphite and non-graphitic carbon at high pressure and temperature. High Pressure Res. 26, 63–69 (2006).
Ohfuji, H. & Kuroki, K. Origin of unique microstructures in nano-polycrystalline diamond synthesized by direct conversion of graphite at static high pressure. J. Mineral. Petrol. Sci. 104, 307–312 (2009).
Fahy, S., Louie, S. G. & Cohen, M. L. Pseudopotential total-energy study of the transition from rhombohedral graphite to diamond. Phys. Rev. B 34, 1191–1199 (1986).
Fahy, S., Louie, S. G. & Cohen, M. L. Theoretical total-energy study of the transformation of graphite into hexagonal diamond. Phys. Rev. B 35, 7623–7626 (1987).
Tateyama, Y., Ogitsu, T., Kusakabe, K. & Tsuneyuki, S. Constant-pressure first-principles studies on the transition states of the graphite–diamond transformation. Phys. Rev. B 54, 14994–15001 (1996).
Scandolo, S., Bernasconi, M., Chiarotti, G. L., Focher, P. & Tosatti, E. Pressure-induced transformation path of graphite to diamond. Phys. Rev. Lett. 74, 4015–4018 (1995).
Zipoli, F., Bernasconi, M. & Martonak, R. Constant pressure reactive molecular dynamics simulations of phase transitions under pressure: The graphite to diamond conversion revisited. Eur. Phys. J. B 39, 41–47 (2004).
Khaliullin, R. Z., Eshet, H., Kühne, T. D., Behler, J. & Parrinello, M. Graphite–diamond phase coexistence study employing a neural-network mapping of the ab initio potential energy surface. Phys. Rev. B 81, 100103 (2010).
Bundy, F. P., Strong, H. M., Bovenkerk, H. P. & Wentorf, R. H. Diamond–graphite equilibrium line from growth and graphitization of diamond. J. Chem. Phys. 35, 383–391 (1961).
Bartok, A. P., Payne, M. C., Kondor, R. & Csanyi, G. Gaussian approximation potentials: The accuracy of quantum mechanics, without the electrons. Phys. Rev. Lett. 104, 136403 (2010).
Mundy, C. J. et al. Ultrafast transformation of graphite to diamond: An ab initio study of graphite under shock compression. J. Chem. Phys. 128, 184701 (2008).
Erskine, D. J. & Nellis, W. J. Shock-induced martensitic phase-transformation of oriented graphite to diamond. Nature 349, 317–319 (1991).
Erskine, D. J. & Nellis, W. J. Shock-induced martensitic-transformation of highly oriented graphite to diamond. J. Appl. Phys. 71, 4882–4886 (1992).
Vanderbilt, D. & Louie, S. G. Total energies of diamond (111) surface reconstructions by a linear combination of atomic orbitals method. Phys. Rev. B 30, 6118–6130 (1984).
Zerilli, F. J. & Jones, H. D. Surface energy and the size of diamond crystals. AIP Conf. Proc. 370, 163–166 (1996).
Bradley, R. S. Effect of pressure on rate of solid reactions, with special reference to diamond synthesis. J. Inorg. Nucl. Chem. 33, 1969–1973 (1971).
Deryagin, B. V. & Fedoseev, D. V. Phase-transitions and nucleation in diamond and graphite. Bull. Acad. Sci. USSR Div. Chem. Sci. 28, 1106–1109 (1979).
Behler, J. & Parrinello, M. Generalized neural-network representation of high-dimensional potential-energy surfaces. Phys. Rev. Lett. 98, 146401 (2007).
Eshet, H., Khaliullin, R. Z., Kühne, T. D., Behler, J. & Parrinello, M. Ab initio quality neural-network potential for sodium. Phys. Rev. B 81, 184107 (2010).
Yagi, T., Utsumi, W., Yamakata, M., Kikegawa, T. & Shimomura, O. High-pressure in situ X-ray-diffraction study of the phase-transformation from graphite to hexagonal diamond at room-temperature. Phys. Rev. B 46, 6031–6039 (1992).
Chu, Y. A., Moran, B., Reid, A. C. E. & Olson, G. B. A model for nonclassical nucleation of solid–solid structural phase transformations. Metall. Mater. Trans. A 31, 1321–1331 (2000).
Eshelby, J. D. The determination of the elastic field of an ellipsoidal inclusion, and related problems. Proc. R. Soc. Lond. A 241, 376–396 (1957).
Le Guillou, C., Brunet, F., Irifune, T., Ohfuji, H. & Rouzaud, J. N. Nanodiamond nucleation below 2273 K at 15 GPa from carbons with different structural organizations. Carbon 45, 636–648 (2007).
Suarez-Martinez, I., Savini, G., Haffenden, G., Campanera, J. M. & Heggie, M. I. Dislocations of Burgers vector c/2 in graphite. Phys. Status Solidi C 4, 2958–2962 (2007).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Von Lilienfeld, O. A., Tavernelli, I., Rothlisberger, U. & Sebastiani, D. Optimization of effective atom centered potentials for London dispersion forces in density functional theory. Phys. Rev. Lett. 93, 153004 (2004).
Acknowledgements
The authors would like to thank G. Tribello for reading the manuscript and M. Ceriotti for discussions. This work was supported by the European Research Council (ERC-2009-AdG-247075). J.B. is grateful for financial support from the FCI and the Deutsche Forschungsgemeinschaft. T.D.K. acknowledges support by the Graduate School of Excellence Mainz. Our thanks are also due to the Swiss National Supercomputing Centre and High Performance Computing Group of ETH Zürich for computer time.
Author information
Authors and Affiliations
Contributions
R.Z.K. conceived the study, implemented the NN code, designed the NN potential, carried out simulations, analysed results and wrote the manuscript; H.E. designed and implemented the NN code, carried out simulations, contributed to the development of the NN potential and analysed results; T.D.K. analysed results and supervised the simulations; J.B. contributed to the implementation of the NN code and supervised the construction of the NN potential; M.P. conceived the study, analysed results, edited the manuscript and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 969 kb)
Rights and permissions
About this article
Cite this article
Khaliullin, R., Eshet, H., Kühne, T. et al. Nucleation mechanism for the direct graphite-to-diamond phase transition. Nature Mater 10, 693–697 (2011). https://doi.org/10.1038/nmat3078
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3078
This article is cited by
-
Active machine learning model for the dynamic simulation and growth mechanisms of carbon on metal surface
Nature Communications (2024)
-
Driving forces for ultrafast laser-induced sp2 to sp3 structural transformation in graphite
npj Computational Materials (2023)
-
Atomic-scale view of the photoinduced structural transition to form sp3-like bonded order phase in graphite
Scientific Reports (2023)
-
Enhancement of short/medium-range order and thermal conductivity in ultrahard sp3 amorphous carbon by C70 precursor
Nature Communications (2023)
-
Dynamics of the fcc-to-bcc phase transition in single-crystalline PdCu alloy nanoparticles
Nature Communications (2023)