Abstract
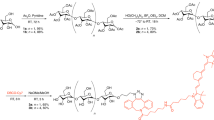
The diagnosis of bacterial infections remains a major challenge in medicine. Although numerous contrast agents have been developed to image bacteria, their clinical impact has been minimal because they are unable to detect small numbers of bacteria in vivo, and cannot distinguish infections from other pathologies such as cancer and inflammation1,2,3,4,5,6,7. Here, we present a family of contrast agents, termed maltodextrin-based imaging probes (MDPs), which can detect bacteria in vivo with a sensitivity two orders of magnitude higher than previously reported, and can detect bacteria using a bacteria-specific mechanism that is independent of host response and secondary pathologies. MDPs are composed of a fluorescent dye conjugated to maltohexaose, and are rapidly internalized through the bacteria-specific maltodextrin transport pathway8,9,10,11, endowing the MDPs with a unique combination of high sensitivity and specificity for bacteria. Here, we show that MDPs selectively accumulate within bacteria at millimolar concentrations, and are a thousand-fold more specific for bacteria than mammalian cells. Furthermore, we demonstrate that MDPs can image as few as 105 colony-forming units in vivo and can discriminate between active bacteria and inflammation induced by either lipopolysaccharides or metabolically inactive bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bettegowda, C. et al. Imaging bacterial infections with radiolabeled 1-(2′-deoxy-2′-fluoro-β-D-arabinofuranosyl)-5-iodouracil. Proc. Natl Acad. Sci. USA 102, 1145–1150 (2005).
Leevy, W. M. et al. Optical imaging of bacterial infection in living mice using a fluorescent near-infrared molecular probe. J. Am. Chem. Soc. 128, 16476–16477 (2006).
Smith, B. A. et al. Optical imaging of mammary and prostate tumors in living animals using a synthetic near infrared zinc(II)-dipicolylamine probe for anionic cell surfaces. J. Am. Chem. Soc. 132, 67–69 (2010).
Welling, M. M., Paulusma-Annema, A., Balter, H. S., Pauwels, E. K. & Nibbering, P. H. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur. J. Nucl. Med. 27, 292–301 (2000).
Mahfouz, T. et al. 18F-fluorodeoxyglucose positron emission tomography contributes to the diagnosis and management of infections in patients with multiple myeloma: A study of 165 infectious episodes. J. Clin. Oncol. 23, 7857–7863 (2005).
Leevy, W. M. et al. Noninvasive optical imaging of Staphylococcus aureus bacterial infection in living mice using a Bis-dipicolylamine-Zinc(II) affinity group conjugated to a near-infrared fluorophore. Bioconjug. Chem. 19, 686–692 (2008).
Rouzet, F. et al. Technetium 99m-labeled annexin V scintigraphy of platelet activation in vegetations of experimental endocarditis. Circulation 117, 781–789 (2008).
Boos, W. & Shuman, H. Maltose/maltodextrin system of Escherichia coli: Transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 62, 204–229 (1998).
Gopal, S. et al. Maltose and maltodextrin utilization by Listeria monocytogenes depend on an inducible ABC transporter which is repressed by glucose. PLoS ONE 5, e10349 (2010).
Oldham, M. L., Khare, D., Quiocho, F. A., Davidson, A. L. & Chen, J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature 450, 515–521 (2007).
Brass, J. M., Bauer, K., Ehmann, U. & Boos, W. Maltose-binding protein does not modulate the activity of maltoporin as a general porin in Escherichia coli. J. Bacteriol. 161, 720–726 (1985).
Lipsky, B. A., Itani, K. & Norden, C. Treating foot infections in diabetic patients: A randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin. Infect. Dis. 38, 17–24 (2004).
Reiber, G. E., Pecoraro, R. E. & Koepsell, T. D. Risk factors for amputation in patients with diabetes mellitus. A case-control study. Ann. Intern. Med. 117, 97–105 (1992).
Moore, E. H. Atypical mycobacterial infection in the lung: CT appearance. Radiology 187, 777–782 (1993).
Erasmus, J. J., McAdams, H. P., Farrell, M. A. & Patz, E. F. Jr Pulmonary nontuberculous mycobacterial infection: Radiologic manifestations. Radiographics 19, 1487–1505 (1999).
Dahl, M. K. & Manson, M. D. Interspecific reconstitution of maltose transport and chemotaxis in Escherichia coli with maltose-binding protein from various enteric bacteria. J. Bacteriol. 164, 1057–1063 (1985).
Reuss, R. et al. Intracellular delivery of carbohydrates into mammalian cells through swelling-activated pathways. J. Membr. Biol. 200, 67–81 (2004).
Line, B. R., Weber, P. B., Lukasiewicz, R. & Dansereau, R. N. Reduction of background activity through radiolabeling of antifibrin Fab′ with 99mTc-dextran. J. Nucl. Med. 41, 1264–1270 (2000).
Demko, Z. P. & Sharpless, K. B. A click chemistry approach to tetrazoles by Huisgen 1,3-dipolar cycloaddition: Synthesis of 5-acyltetrazoles from azides and acyl cyanides. Angew. Chem. Int. Ed. Engl. 41, 2113–2116 (2002).
Tornoe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Dippel, R. & Boos, W. The maltodextrin system of Escherichia coli: Metabolism and transport. J. Bacteriol. 187, 8322–8331 (2005).
Freundlieb, S., Ehmann, U. & Boos, W. Facilitated diffusion of p-nitrophenyl-alpha-D-maltohexaoside through the outer membrane of Escherichia coli. Characterization of LamB as a specific and saturable channel for maltooligosaccharides. J. Biol. Chem. 263, 314–320 (1988).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006).
Reid, G. Biofilms in infectious disease and on medical devices. Int. J. Antimicrob. Agents 11, 223–226 (1999).
Author, A. N. Panel discussion on biofilms in urinary tract infection. Int. J. Antimicrob. Agents 11, 237–239 (1999).
Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nature Rev. Microbiol. 2, 95–108 (2004).
Kolodkin-Gal, I. et al. D-amino acids trigger biofilm disassembly. Science 328, 627–629 (2010).
Dehoux, M. J., van Beneden, R. P., Fernandez-Celemin, L., Lause, P. L. & Thissen, J. P. Induction of MafBx and Murf ubiquitin ligase mRNAs in rat skeletal muscle after LPS injection. FEBS Lett. 544, 214–217 (2003).
Luo, G., Niesel, D. W., Shaban, R. A., Grimm, E. A. & Klimpel, G. R. Tumor necrosis factor alpha binding to bacteria: evidence for a high-affinity receptor and alteration of bacterial virulence properties. Infect. Immun. 61, 830–835 (1993).
Larson, T. J., Ludtke, D. N. & Bell, R. M. sn-Glycerol-3-phosphate auxotrophy of plsB strains of Escherichia coli: evidence that a second mutation, plsX, is required. J. Bacteriol. 160, 711–717 (1984).
Acknowledgements
This project has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000043C, NSF-BES-0546962 Career Award (N.M.) and NIH RO1 HL096796-01 (N.M.).
Author information
Authors and Affiliations
Contributions
X.N. synthesized and characterized MDP-1 and MDP-2, designed and analysed experiments, and wrote the manuscript. S.L. designed, carried out and analysed experiments, and contributed to the writing of the manuscript. Z.W. performed MS experiments to characterize all intermediates and final products and proof read the manuscript. D.K. carried out in vitro experiments. B.S. prepared biofilms and performed confocal laser scanning microscopy. E.G. supervised the preparation of biofilms and proof read the manuscript. N.M. designed and supervised the project and contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ning, X., Lee, S., Wang, Z. et al. Maltodextrin-based imaging probes detect bacteria in vivo with high sensitivity and specificity. Nature Mater 10, 602–607 (2011). https://doi.org/10.1038/nmat3074
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3074
This article is cited by
-
Engineered live bacteria as disease detection and diagnosis tools
Journal of Biological Engineering (2023)
-
In vivo bioluminescence imaging of natural bacteria within deep tissues via ATP-binding cassette sugar transporter
Nature Communications (2023)
-
Activatable near-infrared probes for the detection of specific populations of tumour-infiltrating leukocytes in vivo and in urine
Nature Biomedical Engineering (2023)
-
Bacteria loaded with glucose polymer and photosensitive ICG silicon-nanoparticles for glioblastoma photothermal immunotherapy
Nature Communications (2022)
-
Bacteria eat nanoprobes for aggregation-enhanced imaging and killing diverse microorganisms
Nature Communications (2022)