Abstract
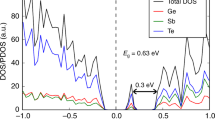
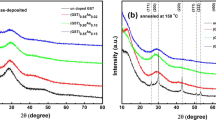
Phase-change optical memories are based on the astonishingly rapid nanosecond-scale crystallization of nanosized amorphous ‘marks’ in a polycrystalline layer. Models of crystallization exist for the commercially used phase-change alloy Ge2Sb2Te5 (GST), but not for the equally important class of Sb–Te-based alloys. We have combined X-ray diffraction, extended X-ray absorption fine structure and hard X-ray photoelectron spectroscopy experiments with density functional simulations to determine the crystalline and amorphous structures of Ag3.5In3.8Sb75.0Te17.7 (AIST) and how they differ from GST. The structure of amorphous (a-) AIST shows a range of atomic ring sizes, whereas a-GST shows mainly small rings and cavities. The local environment of Sb in both forms of AIST is a distorted 3+3 octahedron. These structures suggest a bond-interchange model, where a sequence of small displacements of Sb atoms accompanied by interchanges of short and long bonds is the origin of the rapid crystallization of a-AIST. It differs profoundly from crystallization in a-GST.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Meinders, E. R., Mijiritskii, A. V., van Pieterson, L. & Wuttig, M. Optical Data Storage Vol. 4 (Philips Research Book Series, Springer, 2006).
Yamada, N., Ohno, E., Nishiuchi, K., Akahira, N. & Takao, M. Rapid phase transitions of GeTe–Sb2Te3 pseudobinary amorphous thin films for an optical disk memory. J. Appl. Phys. 69, 2849–2856 (1991).
Matsunaga, T. & Yamada, N. Crystallographic studies on high-speed phase-change materials used for rewritable optical recording disks. Jpn J. Appl. Phys. 43, 4704–4712 (2004).
Elliott, S. R. Physics of Amorphous Materials (Longman, 1984).
Lee, B-S. et al. Observation of the role of subcritical nuclei in crystallization of a glassy solid. Science 326, 980–984 (2009).
Fukuyama, Y. et al. Time-resolved investigation of nanosecond crystal growth in rapid phase-change materials—correlation with the recording speed of digital versatile disc media. Appl. Phys. Exp. 1, 045001 (2008).
Wuttig, M. & Yamada, N. Phase-change materials for rewriteable data storage. Nature Mater. 6, 824–832 (2007).
Matsunaga, T., Umetani, Y. & Yamada, N. Structural study of a Ag3.4In3.7Sb76.4Te16.5 quadruple compound utilized for phase-change optical disks. Phys. Rev. B 64, 184116 (2001).
Akola, J. et al. Experimentally constrained density functional calculations of the amorphous structure of the prototypical phase-change material Ge2Sb2Te5 . Phys. Rev. B 80, 020201(R) (2009).
Kohara, S. et al. Structural basis for the fast phase change of Ge2Sb2Te5: Ring statistics analogy between the crystal and amorphous states. Appl. Phys. Lett. 89, 201910 (2006).
Lee, M. L., Shi, L. P., Tian, Y. T., Gan, C. L. & Miao, X. S. Crystallization behavior of Sb70Te30 and Ag3In5Sb60Te32 chalcogenide materials for optical media applications. Phys. Status Solidi a 205, 340–344 (2008).
Akola, J. & Jones, R. O. Structural phase transitions on the nanoscale: The crucial pattern in the phase change materials Ge2Sb2Te5 and GeTe. Phys. Rev. B 76, 235201 (2007).
Akola, J. & Jones, R. O. Density functional study of amorphous, liquid, and crystalline Ge2Sb2Te5: Homopolar bonds and/or AB alternation? J. Phys. Condens. Matter 20, 365103 (2008).
Tashiro, H. et al. Structural analysis of Ag–In–Sb–Te phase-change material. Jpn J. Appl. Phys. 41, 3758–3759 (2002).
Kim, J-J. et al. Electronic structure of amorphous and crystalline (GeTe)1−x(Sb2Te3)x investigated using hard X-ray photoemission spectroscopy. Phys. Rev. B 76, 115124 (2007).
Hoffmann, R. Solids and Surfaces: A Chemist’s View of Bonding in Extended Structures (Wiley-VCH, 1989).
Shportko, K. et al. Resonant bonding in crystalline phase-change materials. Nature Mater. 7, 653–658 (2008).
Huang, B. & Robertson, J. Bonding origin of optical contrast in phase-change memory materials. Phys. Rev. B 81, 081204 (2010).
Gronert, S. Gas phase studies of the competition between substitution and elimination reactions. Acc. Chem. Res. 36, 848–857 (2003).
Mikosch, J. et al. Imaging nucleophilic substitution dynamics. Science 319, 183–186 (2008).
Binnemans, K. Ionic liquid crystals. Chem. Rev. 105, 4148–4204 (2005).
Njoroge, W. K. & Wuttig, M. Crystallization kinetics of sputter-deposited amorphous AgInSbTe films. J. Appl. Phys. 90, 3816–3821 (2001).
Shakhvorostov, D. et al. Evidence of electronic gap-driven metal–semiconductor transition in phase change materials. Proc. Natl Acad. Sci. USA 106, 10907–10911 (2009).
Cooper, A. R. Zachariasen’s rules, Madelung constant, and network topology. Phys. Chem. Glasses 19, 60–68 (1978).
Ziman, J. M. Models of Disorder (Cambridge Univ. Press, 1979).
Her, U-C., Chen, H. & Hsu, Y-S. Effects of Ag and In addition on the optical properties and crystallization kinetics of eutectic Sb70Te30 phase-change recording film. J. Appl. Phys. 93, 10097–10103 (2003).
Caravati, S., Bernasconi, M., Kühne, T. D., Krack, M. & Parrinello, M. Coexistence of tetrahedral- and octahedral-like sites in amorphous phase change materials. Appl. Phys. Lett. 89, 171906 (2007).
Hegedüs, J. & Elliott, S. R. Microscopic origin of the fast crystallization ability of Ge–Sb–Te phase-change memory materials. Nature Mater. 7, 399–405 (2008).
Kohara, S. et al. Structural studies of disordered materials using high-energy X-ray diffraction from ambient to extreme conditions. J. Phys. Condens. Matter 19, 506101 (2007).
Ankudinov, A. L., Ravel, B., Rehr, J. J. & Conradson, S. D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 58, 7565–7576 (1998).
Taguchi, T., Ozawa, T. & Yashiro, H. REX2000: Yet another XAFS analysis package. Phys. Scr. T115, 205–206 (2005).
Kobayashi, K. et al. High resolution–high energy X-ray photoelectron spectroscopy using third-generation synchrotron radiation source, and its application to Si–high k insulator systems. Appl. Phys. Lett. 83, 1005–1007 (2003).
Shirley, D. A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 5, 4709–4714 (1972).
CPMD version 3.13. © IBM Corporation (1990–2009), © MPI für Festkörperforschung, Stuttgart (1997–2001).
Perdew, J. P. et al. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 100, 136406 (2008).
Troullier, N. & Martins, J. L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 43, 1993–2006 (1991).
Akola, J. & Jones, R. O. Structure of liquid phase change material AgInSbTe from density functional/molecular dynamics simulations. Appl. Phys. Lett. 94, 251905 (2009).
Greben, O., Jóvári, P., Temleitner, L. & Pusztai, L. A new version of the RMC++ reverse Monte Carlo programme, aimed at investigating the structure of covalent glasses. J. Optoelectron. Adv. Mater. 9, 3021–3027 (2007).
Acknowledgements
This work was supported by Core Research for Evolutional Science and Technology (CREST) ‘X-ray pinpoint structural measurement project—Development of the spatial- and time-resolved structural study for nano-materials and devices’ and by the Academy of Finland and the Japan Science and Technology Agency through the Strategic Japanese–Finnish Cooperative Program on ‘Functional materials’. The synchrotron radiation experiments were approved by the Japan Synchrotron Radiation Research Institute (proposal Nos 2007A1223, 2008A1409 and 2009A12386), and all calculations were carried out on the Jugene (IBM BlueGene/P) and Juropa (Xeon 5570) computers in the Forschungszentrum Jülich with grants from the John von Neumann Institute for Computing and the Forschungszentrum Jülich. We thank N. Yasuda and Y. Fukuyama for assistance in the density estimation measurement and H-P. Komsa for providing the initial 648-atom system coordinates for crystalline AIST.
Author information
Authors and Affiliations
Contributions
The experiments/calculations were carried out and analysed as follows: sample preparation, R.K., T.M., N.Y.; high-energy XRD and RMC, S.K., M.T.; EXAFS, T.H., S.K., T.M.; XPS, E.I., K.K., T.M.; DF-MD, J.A., R.O.J. The manuscript was planned by J.A., R.O.J., S.K., T.M. and N.Y. and written by R.O.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplemntary Information (PDF 870 kb)
Rights and permissions
About this article
Cite this article
Matsunaga, T., Akola, J., Kohara, S. et al. From local structure to nanosecond recrystallization dynamics in AgInSbTe phase-change materials. Nature Mater 10, 129–134 (2011). https://doi.org/10.1038/nmat2931
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2931
This article is cited by
-
Multiscale simulations of growth-dominated Sb2Te phase-change material for non-volatile photonic applications
npj Computational Materials (2023)
-
Thermodynamic Modeling of the Cu-Sb-Se System
Journal of Phase Equilibria and Diffusion (2023)
-
Hypervalency in amorphous chalcogenides
Nature Communications (2022)
-
The potential of chemical bonding to design crystallization and vitrification kinetics
Nature Communications (2021)
-
Ab initio molecular dynamics and materials design for embedded phase-change memory
npj Computational Materials (2021)