Abstract
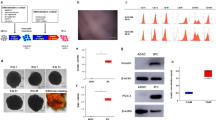
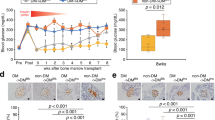
To explore induced islet neogenesis in the liver as a strategy for the treatment of diabetes, we used helper-dependent adenovirus (HDAD) to deliver the pancreatic duodenal homeobox-1 gene (Ipf1; also known as Pdx-1) to streptozotocin (STZ)-treated diabetic mice. HDAD is relatively nontoxic as it is devoid of genes encoding viral protein. Mice treated with HDAD-Ipf1 developed fulminant hepatitis, however, because of the exocrine-differentiating activity of Ipf1. The diabetes of STZ mice was partially reversed by HDAD-mediated transfer of NeuroD (Neurod), a factor downstream of Ipf1, and completely reversed by a combination of Neurod and betacellulin (Btc), without producing hepatitis. Treated mice were healthy and normoglycemic for the duration of the experiment (>120 d). We detected in the liver insulin and other islet-specific transcripts, including proinsulin-processing enzymes, β-cell–specific glucokinase and sulfonylurea receptor. Immunocytochemistry detected the presence of insulin, glucagon, pancreatic polypeptide and somatostatin-producing cells organized into islet clusters; immuno-electron microscopy showed typical insulin-containing granules. Our data suggest that Neurod-Btc gene therapy is a promising regimen to induce islet neogenesis for the treatment of insulin-dependent diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylur as or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998).
Stratton, I.M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321, 405–412 (2000).
Shapiro, A.M. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–208 (2000).
Paty, B.W., Ryan, E.A., Shapiro, A.M., Lakey, J.R. & Robertson, R.P. Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes 51, 3428–3434 (2002).
Dong, H. & Woo, S.L. Hepatic insulin production for type 1 diabetes. Trends Endocrinol. Metab. 12, 441–446 (2001).
Lee, H.C., Kim, S.J., Kim, K.S., Shin, H.C. & Yoon, J.W. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature 408, 483–488 (2000).
Auricchio, A. et al. Constitutive and regulated expression of processed insulin following in vivo hepatic gene transfer. Gene Ther. 9, 963–971 (2002).
Ferber, S. et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 6, 568–572 (2000).
Kochanek, S. High-capacity adenoviral vectors for gene transfer and somatic gene therapy. Hum. Gene Ther. 10, 2451–2459 (1999).
Morral, N. et al. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of α1-antitrypsin with negligible toxicity. Hum. Gene Ther. 9, 2709–2716 (1998).
Oka, K. et al. Long-term stable correction of LDL receptor-deficient mice with a helper-dependent adenoviral vector expressing the VLDL receptor. Circulation 103, 1274–1281 (2001).
Kim, I.-H., Józkowicz, A., Piedra, P.A., Oka, K. & Chan, L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc. Natl. Acad. Sci. USA 98, 13282–13287 (2001).
O'Neal, W.K. et al. Toxicological comparison of E2a-deleted and first-generation adenoviral vectors expressing α1-antitrypsin after systemic delivery. Hum. Gene Ther. 9, 1587–1598 (1998).
Lozier, J.N., Metzger, M.E., Donahue, R.E. & Morgan, R.A. Adenovirus-mediated expression of human coagulation foactor IX in the rhesus macaque is associated with dose-limiting toxicity. Blood 94, 3968–3975 (1999).
Yang, L. et al. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc. Natl. Acad. Sci. USA 99, 8078–8083 (2002).
Gu, G., Dubauskaite, J. & Melton, D.A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002).
Pin, C.L., Rukstalis, J.M., Johnson, C. & Konieczny, S.F. The bHLH transcription factor mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J. Cell Biol. 155, 519–530 (2001).
Naya, F.J. et al. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/NeuroD-deficient mice. Genes Dev. 11, 2323–2334 (1997).
Shing, Y. et al. Betacellulin: a mitogen from pancreatic beta cell tumors. Science 259, 2681–2689 (1993).
Kojima, H. et al. Combined expression of pancreatic duodenal homeobox 1 and islet factor 1 induces immature enterocytes to produce insulin. Diabetes 51, 1398–1408 (2002).
Ryan, E.A. et al. Successful islet transplantation. Continued insulin reverse provides long-term glycemic control. Diabetes 51, 2148–2157 (2002).
Watari, N. & Hotta, Y. Three-dimensional structure of crystalline insuling granules as analyzed by high-voltage electron microscopy and computer calculation. in Endocrine Gut and Pancreas (ed. Fujita, T.) 179–184 (Elsevier Science Publishing Co., Amsterdam, 1975).
Halban, P.A., Kahn, S.E., Lernmark, A. & Rhodes, C.J. Gene and cell-replacement therapy in the treatment of type 1 diabetes. How high must the standards be set? Diabetes 50, 2181–2191 (2001).
Yoon, J.W. & Jun, H.S. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol. Med. 8, 62–68 (2002).
Theise, N.D. et al. The canals of hering and hepatic stem cells in humans. Hepatology 30, 1425–1433 (1999).
Mizushima, S. & Nagata, S. pEF-BOS, a powerful mammalian expression vector. Nucl. Acids Res. 18, 5322 (1990).
Beale, E.G., Clouthier, D.E. & Hammer, R.E. Cell-specific expression of cytosolic phosphoenolpyruvate carboxykinase in transgenic mice. FASEB J. 6, 3330–3337 (1992).
Parks, R.J. et al. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 93, 13565–13570 (1996).
Kojima, H., Harano, Y., Kosugi, K., Nakano, T. & Shigeta, Y. A suppressive role of c-kinase for the stimulation of hepatic ketogenesis by glucagon and epinephrine. FEBS Lett. 201, 271–276 (1986).
Fujimiya, M., McIntosh, C.H., Kimura, H. & Kwok, Y.N. Effect of carbachol on luminal release of somatostatin from isolated perfused rat duodenum. Neurosci. Lett. 145, 229–233 (1992).
Fujimiya, M., Okumiya, K. & Kuwahara, R. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem. Cell Biol. 108, 105–113 (1997).
Acknowledgements
We thank R. Kikkawa, A. Kashiwagi and Y. Shigeta for interest and support; K. Oka, E.A. Nour, B. Thompson and M. Nishimura for technical assistance; and the Central Research Laboratory of Shiga University of Medical Science for technical support in fluorescence and electron microscopy. This work was supported by the Rutherford Chair in Diabetes Research at St. Luke's Episcopal Hospital and Baylor College of Medicine (to L.C.) and grants (HL-51586, HL-16512 and HL-59314) from the National Institutes of Health and the Japan Foundation for Aging and Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Kojima, H., Fujimiya, M., Matsumura, K. et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med 9, 596–603 (2003). https://doi.org/10.1038/nm867
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm867
This article is cited by
-
Emerging therapeutic options in the management of diabetes: recent trends, challenges and future directions
International Journal of Obesity (2023)
-
Regenerative approaches to preserve pancreatic β-cell mass and function in diabetes pathogenesis
Endocrine (2022)
-
Delivery of transcription factors as modulators of cell differentiation
Drug Delivery and Translational Research (2021)
-
Liver to Pancreas Transdifferentiation
Current Diabetes Reports (2019)
-
How, When, and Where Do Human β-Cells Regenerate?
Current Diabetes Reports (2019)