Abstract
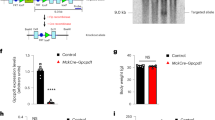
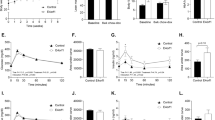
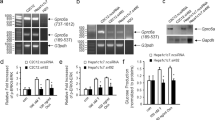
GLUT4, the insulin-responsive glucose transporter, plays an important role in postprandial glucose disposal. Altered GLUT4 activity is suggested to be one of the factors responsible for decreased glucose uptake in muscle and adipose tissue in obesity and diabetes. To assess the effect of GLUT4 expression on whole-body glucose homeostasis, we disrupted the murine GLUT4 gene by homologous recombination. Male mice heterozygous for the mutation (GLUT+/−) exhibited a decrease in GLUT4 expression in adipose tissue and skeletal muscle. This decrease in GLUT4 expression did not result in obesity but led to increased serum glucose and insulin, reduced muscle glucose uptake, hypertension, and diabetic histopathologies in the heart and liver similar to those of humans with non-insulin-dependent diabetes mellitus (NIDDM). The male GLUT4+/− mice represent a good model for studying the development of NIDDM without the complications associated with obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
DeFronzo, R.A. The triumvirate: β-cell, muscle, liver: A collusion responsible for NIDDM (Lilly Lecture 1987). Diabetes 37, 667–687 (1988).
Bennett, P.H. Epidemiology of diabetes mellitus. in Diabetes Mellitus: Theory and Practice, 4th edn. (eds. Rifkin, H. & Porte, D., Jr.) 357–377 (Elsevier Science, New York, 1991).
Mueckler, M. Facilitate glucose transporters. Eur. J. Biochem. 219, 713–725 (1994).
Wright, E.W., Turk, E., Zabel, B., Mundilos, S. & Dyer, J. Molecular genetics of intestinal glucose transport. J. Clin. Invest. 88, 1435–1440 (1991).
Charron, M.J., Brosius, F.C., III, Alper, S.L., & Lodish, H.F., A glucose transport protein expressed predominantly in insulin-responsive tissues. Proc. Natl. Acad. Sci. USA 86, 2535–2539 (1989).
DeFronzo, R.A. et al. The effect of insulin on the disposal of intravenous glucose: Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30, 1000–1007 (1981).
Hirshman, M.F., Goodyear, L.J., Wardzala, L.J., Horton, E.D. & Horton, E.S. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J. Biol. Chem. 265, 987–991 (1990).
Klip, A. et al. Recruitment of GLUT4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem. Biophys. Res. Commun. 172, 728–736 (1990).
Slot, J.W., Geuze, H.J., Gigengack, S., James, D.E. & Lienhard, G.E. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc. Natl. Acad. Sci. USA 88, 7815–7819 (1991).
Bonadonna, R.C. et al. Glucose transport in human skeletal muscle: The in vivo response to insulin. Diabetes 42, 191–198 (1993).
Chan, T.M. & Tatoyan, A. Glucose transport and metabolism in the perfused hindquarters of lean and obese-hyperglycemic (db/db) mice: Effects of insulin and electrical stimulation. Biochim. Biophys. Acta 798, 325–332 (1984).
Penicaud, L. et al. Development of obesity in Zucker rats: Early insulin resistance in muscles but normal sensitivity in white adipose tissue. Diabetes 36, 626–631 (1987).
Sherman, W.M., Katz, A.L., Cutler, C.L., Withers, R.T. & Ivy, J.L. Glucose transport: Locus of muscle insulin resistance in obese Zucker rats. Am. J. Physiol. 255, E374–E382 (1988).
Zierath, J.R. et al. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia 39, 1180–1189 (1996).
Kahn, B.B. Alterations in glucose transporter expression and function in diabetes: Mechanisms for insulin resistance. J. Cell Biochem. 48, 122–128 (1992).
Katz, E.B., Stenbit, A.E., Hatton, K., DePinho, R. & Charron, M.J. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377, 151–155 (1995).
Coleman, D.L. Obese and diabetes: Two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14, 141–148 (1978).
Wyse, B.M. & Dulin, W.E. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia 6, 268–273 (1970).
York, D.A., Steinke, J. & Bray, G.A. Hyperinsulinemia and insulin resistance in genetically obese rats. Metab. Clin. Exp. 21, 277–284 (1972).
Factor, S.M., Minase, T. & Sonnenblick, E.H. Clinical and morphological features of human hypertensive-diabetic cardiomyopathy. Am. Heart J. 99, 446–458 (1980).
Van Hoeven, K.H. & Factor, S.M. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 82, 848–855 (1990).
Stenbit, A.E. et al. Diverse effects of Glut 4 ablation on glucose uptake and glycogen synthesis in red and white skeletal muscle. J. Clin. Invest. 98, 629–634 (1996).
Bruning, J.C. et al. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88, 561–572 (1997).
De Meyts, P. The diabetogenes concept of NIDDM. in New Concepts in the Pathogenesis of NIDDM (eds. Ostenson, C.G. et al.) 89–100 (Plenum, New York, 1993).
Katz, E.B., Burcelin, R., Tsao, T.S., Stenbit, A.E. & Charron, M.J. The metabolic consequences of altered glucose transporter expression in transgenic mice. J. Mol. Med. 74, 639–652 (1996).
Horton, E.S. & Jeanrenaud, B. Obesity and diabetes mellitus. in Diabetes Mellitus: Theory and Practice, 4th edn. (eds. Rifkin, H. & Porte, D., Jr.) 457–465 (Elsevier Science, New York, 1991).
Cushman, S.W. & Salans, L.B. Determinations of adipose cell size and number in suspensions of isolated rat and human adipose cells. J. Lipid Res. 19, 269–273 (1978).
Kahn, B.B., Charron, M.J. & Lodish, H.F. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J. Clin. Invest. 84, 404–411 (1991).
Ioffe, E. et al. WW6: An embryonic stem cell line with an inert genetic marker that can be traced in chimeras. Proc. Natl. Acad. Sci. USA 92, 7357–7361 (1995).
Baldini, G., Holman, R., Charron, M.J. & Lodish, H.F. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT4 to the plasma membrane in alpha-toxin-permeabilized rat adipose cells. J. Biol Chem. 266, 4037–4040 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stenbit, A., Tsao, TS., Li, J. et al. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat Med 3, 1096–1101 (1997). https://doi.org/10.1038/nm1097-1096
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm1097-1096
This article is cited by
-
Combined transcriptome and metabolome analysis reveals breed-specific regulatory mechanisms in Dorper and Tan sheep
BMC Genomics (2024)
-
Effects of short-term endurance and strength exercise in the molecular regulation of skeletal muscle in hyperinsulinemic and hyperglycemic Slc2a4+/− mice
Cellular and Molecular Life Sciences (2023)
-
Genome-wide association study and functional characterization identifies candidate genes for insulin-stimulated glucose uptake
Nature Genetics (2023)
-
Linking metabolic dysfunction with cardiovascular diseases: Brn-3b/POU4F2 transcription factor in cardiometabolic tissues in health and disease
Cell Death & Disease (2021)
-
Baicalin and its aglycone: a novel approach for treatment of metabolic disorders
Pharmacological Reports (2020)