Abstract
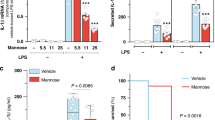
D-mannose, a C-2 epimer of glucose, exists naturally in many plants and fruits, and is found in human blood at concentrations less than one-fiftieth of that of glucose. However, although the roles of glucose in T cell metabolism, diabetes and obesity are well characterized, the function of D-mannose in T cell immune responses remains unknown. Here we show that supraphysiological levels of D-mannose safely achievable by drinking-water supplementation suppressed immunopathology in mouse models of autoimmune diabetes and airway inflammation, and increased the proportion of Foxp3+ regulatory T cells (Treg cells) in mice. In vitro, D-mannose stimulated Treg cell differentiation in human and mouse cells by promoting TGF-β activation, which in turn was mediated by upregulation of integrin αvβ8 and reactive oxygen species generated by increased fatty acid oxidation. This previously unrecognized immunoregulatory function of D-mannose may have clinical applications for immunopathology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Etchison, J.R. & Freeze, H.H. Enzymatic assay of D-mannose in serum. Clin. Chem. 43, 533–538 (1997).
Schneider, A. et al. Successful prenatal mannose treatment for congenital disorder of glycosylation-Ia in mice. Nat. Med. 18, 71–73 (2011).
Alton, G. et al. Direct utilization of mannose for mammalian glycoprotein biosynthesis. Glycobiology 8, 285–295 (1998).
de Lonlay, P. & Seta, N. The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib. Biochim. Biophys. Acta 1792, 841–843 (2009).
Michaels, E.K., Chmiel, J.S., Plotkin, B.J. & Schaeffer, A.J. Effect of D-mannose and D-glucose on Escherichia coli bacteriuria in rats. Urol. Res. 11, 97–102 (1983).
Kranjčec, B., Papeš, D. & Altarac, S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J. Urol. 32, 79–84 (2014).
Schaeffer, A.J., Chmiel, J.S., Duncan, J.L. & Falkowski, W.S. Mannose-sensitive adherence of Escherichia coli to epithelial cells from women with recurrent urinary tract infections. J. Urol. 131, 906–910 (1984).
Wang, R. & Green, D.R. Metabolic checkpoints in activated T cells. Nat. Immunol. 13, 907–915 (2012).
MacIver, N.J., Michalek, R.D. & Rathmell, J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283 (2013).
Vander Heiden, M.G., Cantley, L.C. & Thompson, C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Sun, C.M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Thornton, A.M. & Shevach, E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188, 287–296 (1998).
Read, S., Malmström, V. & Powrie, F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192, 295–302 (2000).
Huehn, J., Polansky, J.K. & Hamann, A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat. Rev. Immunol. 9, 83–89 (2009).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Chen, W. & Konkel, J.E. Development of thymic Foxp3+ regulatory T cells: TGF-β matters. Eur. J. Immunol. 45, 958–965 (2015).
Konkel, J.E., Jin, W., Abbatiello, B., Grainger, J.R. & Chen, W. Thymocyte apoptosis drives the intrathymic generation of regulatory T cells. Proc. Natl. Acad. Sci. USA 111, E465–E473 (2014).
Derynck, R. & Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Zhang, Y., Feng, X.H. & Derynck, R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature 394, 909–913 (1998).
Liu, Y. et al. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 9, 632–640 (2008).
Nakatsukasa, H. et al. The DNA-binding inhibitor Id3 regulates IL-9 production in CD4+ T cells. Nat. Immunol. 16, 1077–1084 (2015).
Yang, X. et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 18, 1280–1291 (1999).
Tone, Y. et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 9, 194–202 (2008).
Chen, W. & Konkel, J.E. TGF-β and 'adaptive' Foxp3+ regulatory T cells. J. Mol. Cell Biol. 2, 30–36 (2010).
Baecher-Allan, C., Brown, J.A., Freeman, G.J. & Hafler, D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167, 1245–1253 (2001).
Chen, W. & Wahl, S.M. TGF-β: receptors, signaling pathways and autoimmunity. Curr. Dir. Autoimmun. 5, 62–91 (2002).
Massagué, J. & Chen, Y.G. Controlling TGF-β signaling. Genes Dev. 14, 627–644 (2000).
Shi, M. et al. Latent TGF-β structure and activation. Nature 474, 343–349 (2011).
Worthington, J.J. et al. Integrin αvβ8-mediated TGF-β activation by effector regulatory T cells is essential for suppression of T-cell-mediated inflammation. Immunity 42, 903–915 (2015).
Travis, M.A. et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361–365 (2007).
Edwards, J.P., Thornton, A.M. & Shevach, E.M. Release of active TGF-β1 from the latent TGF-β1/GARP complex on T regulatory cells is mediated by integrin β8. J. Immunol. 193, 2843–2849 (2014).
Chen, W., Frank, M.E., Jin, W. & Wahl, S.M. TGF-β released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14, 715–725 (2001).
Amarnath, S., Dong, L., Li, J., Wu, Y. & Chen, W. Endogenous TGF-β activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25− T cells. Retrovirology 4, 57 (2007).
Hildeman, D.A., Mitchell, T., Kappler, J. & Marrack, P. T cell apoptosis and reactive oxygen species. J. Clin. Invest. 111, 575–581 (2003).
Sena, L.A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Bulua, A.C. et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208, 519–533 (2011).
Rosca, M.G. et al. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 61, 2074–2083 (2012).
Seifert, E.L., Estey, C., Xuan, J.Y. & Harper, M.E. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J. Biol. Chem. 285, 5748–5758 (2010).
Anderson, M.S. & Bluestone, J.A. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 23, 447–485 (2005).
Akirav, E.M. et al. Detection of β cell death in diabetes using differentially methylated circulating DNA. Proc. Natl. Acad. Sci. USA 108, 19018–19023 (2011).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 37, S81–S90 (2014).
Walter, D.M. et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J. Immunol. 167, 4668–4675 (2001).
Takaoka, A. et al. A critical role for mouse CXC chemokine(s) in pulmonary neutrophilia during Th type 1-dependent airway inflammation. J. Immunol. 167, 2349–2353 (2001).
Staudt, V. et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33, 192–202 (2010).
Wu, L. & Derynck, R. Essential role of TGF-β signaling in glucose-induced cell hypertrophy. Dev. Cell 17, 35–48 (2009).
Chang, C.H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Travis, M.A. & Sheppard, D. TGF-β activation and function in immunity. Annu. Rev. Immunol. 32, 51–82 (2014).
Davis, J.A. & Freeze, H.H. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim. Biophys. Acta 1528, 116–126 (2001).
Derynck, R. & Akhurst, R.J. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nat. Cell Biol. 9, 1000–1004 (2007).
Mayatepek, E., Schröder, M., Kohlmüller, D., Bieger, W.P. & Nützenadel, W. Continuous mannose infusion in carbohydrate-deficient glycoprotein syndrome type I. Acta Paediatr. 86, 1138–1140 (1997).
Feuerer, M., Shen, Y., Littman, D.R., Benoist, C. & Mathis, D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity 31, 654–664 (2009).
Kasagi, S. et al. In vivo-generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Sci. Transl. Med. 6, 241ra78 (2014).
Zanvit, P. et al. Antibiotics in neonatal life increase murine susceptibility to experimental psoriasis. Nat. Commun. 6, 8424 (2015).
Konkel, J.E. et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat. Immunol. 12, 312–319 (2011).
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, NIDCR. We thank E. Shevach (NIAID, NIH, Bethesda, Maryland, USA) for providing the Itgb8f/fCD4-Cre mice, and C. Benoist and D. Mathis (Harvard Medical School, Boston, Massachusetts, USA) for providing the NOD-Foxp3DTR mice. We also thank the NIDCR flow cytometry core for support.
Author information
Authors and Affiliations
Contributions
D.Z. designed and performed most of the experiments, analyzed and interpreted the data, and contributed to the writing of the manuscript. C.C. and X.J. designed and conducted experiments, analyzed data, and contributed to the writing of the manuscript. S.K., R.W., J.E.K., H.N., P.Z., N.G. and W.J. performed experiments. Q.C., L.S. and Z.-J.C. provided support and/or critical scientific input. W.J.C. conceived of and directed the research, designed the experiments, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
Supplementary Figures 1–15. (PDF 2965 kb)
Rights and permissions
About this article
Cite this article
Zhang, D., Chia, C., Jiao, X. et al. D-mannose induces regulatory T cells and suppresses immunopathology. Nat Med 23, 1036–1045 (2017). https://doi.org/10.1038/nm.4375
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4375
This article is cited by
-
D-mannose promotes the degradation of IDH2 through upregulation of RNF185 and suppresses breast cancer
Nutrition & Metabolism (2024)
-
ACSS2 controls PPARγ activity homeostasis to potentiate adipose-tissue plasticity
Cell Death & Differentiation (2024)
-
PMI-controlled mannose metabolism and glycosylation determines tissue tolerance and virus fitness
Nature Communications (2024)
-
D-Mannose prevents bone loss under weightlessness
Journal of Translational Medicine (2023)
-
D-mannose induces TFE3-dependent lysosomal degradation of EGFR and inhibits the progression of NSCLC
Oncogene (2023)