Abstract
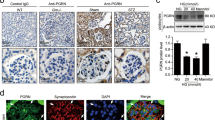
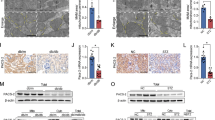
Diabetic nephropathy (DN) is a major cause of end-stage renal disease, and therapeutic options for preventing its progression are limited. To identify novel therapeutic strategies, we studied protective factors for DN using proteomics on glomeruli from individuals with extreme duration of diabetes (ł50 years) without DN and those with histologic signs of DN. Enzymes in the glycolytic, sorbitol, methylglyoxal and mitochondrial pathways were elevated in individuals without DN. In particular, pyruvate kinase M2 (PKM2) expression and activity were upregulated. Mechanistically, we showed that hyperglycemia and diabetes decreased PKM2 tetramer formation and activity by sulfenylation in mouse glomeruli and cultured podocytes. Pkm-knockdown immortalized mouse podocytes had higher levels of toxic glucose metabolites, mitochondrial dysfunction and apoptosis. Podocyte-specific Pkm2-knockout (KO) mice with diabetes developed worse albuminuria and glomerular pathology. Conversely, we found that pharmacological activation of PKM2 by a small-molecule PKM2 activator, TEPP-46, reversed hyperglycemia-induced elevation in toxic glucose metabolites and mitochondrial dysfunction, partially by increasing glycolytic flux and PGC-1α mRNA in cultured podocytes. In intervention studies using DBA2/J and Nos3 (eNos) KO mouse models of diabetes, TEPP-46 treatment reversed metabolic abnormalities, mitochondrial dysfunction and kidney pathology. Thus, PKM2 activation may protect against DN by increasing glucose metabolic flux, inhibiting the production of toxic glucose metabolites and inducing mitochondrial biogenesis to restore mitochondrial function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Breyer, M.D. & Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Discov. 15, 568–588 (2016).
Khan, S.S. & Quaggin, S.E. Therapies on the horizon for diabetic kidney disease. Curr. Diab. Rep. 15, 111 (2015).
United States Renal Data System. 2015 USRDS Annual Data Report (USRDS, 2015).
Brownlee, M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625 (2005).
Nishikawa, T. et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790 (2000).
Dunlop, M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int. Suppl. 77, S3–S12 (2000).
Giacco, F. et al. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 63, 291–299 (2014).
Greene, D.A., Lattimer, S.A. & Sima, A.A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N. Engl. J. Med. 316, 599–606 (1987).
Lee, T.S., Saltsman, K.A., Ohashi, H. & King, G.L. Activation of protein kinase C by elevation of glucose concentration: proposal for a mechanism in the development of diabetic vascular complications. Proc. Natl. Acad. Sci. USA 86, 5141–5145 (1989).
Reidy, K., Kang, H.M., Hostetter, T. & Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Invest. 124, 2333–2340 (2014).
Reddy, M.A. & Natarajan, R. Epigenetics in diabetic kidney disease. J. Am. Soc. Nephrol. 22, 2182–2185 (2011).
Fogo, A.B. The targeted podocyte. J. Clin. Invest. 121, 2142–2145 (2011).
Wang, W. et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 15, 186–200 (2012).
Bolton, W.K. et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am. J. Nephrol. 24, 32–40 (2004).
Frank, R.N. The aldose reductase controversy. Diabetes 43, 169–172 (1994).
PKC-DRS Study Group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes 54, 2188–2197 (2005).
Pergola, P.E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365, 327–336 (2011).
Keenan, H.A. et al. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care 30, 1995–1997 (2007).
Sun, J.K. et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care 34, 968–974 (2011).
Tervaert, T.W. et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 21, 556–563 (2010).
Anastasiou, D. et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 (2011).
Kung, C. et al. Small molecule activation of PKM2 in cancer cells induces serine auxotrophy. Chem. Biol. 19, 1187–1198 (2012).
Nakatsu, D. et al. L-cysteine reversibly inhibits glucose-induced biphasic insulin secretion and ATP production by inactivating PKM2. Proc. Natl. Acad. Sci. USA 112, E1067–E1076 (2015).
Palsson-McDermott, E.M. et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 21, 65–80 (2015).
Geraldes, P. et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 15, 1298–1306 (2009).
Khamaisi, M. et al. PKCδ inhibition normalizes the wound-healing capacity of diabetic human fibroblasts. J. Clin. Invest. 126, 837–853 (2016).
Mima, A. et al. Glomerular VEGF resistance induced by PKCδ/SHP-1 activation and contribution to diabetic nephropathy. FASEB J. 26, 2963–2974 (2012).
Israelsen, W.J. et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell 155, 397–409 (2013).
Varanita, T. et al. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 21, 834–844 (2015).
Kanetsuna, Y. et al. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am. J. Pathol. 170, 1473–1484 (2007).
Nakagawa, T. et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J. Am. Soc. Nephrol. 18, 539–550 (2007).
Sharma, K. Mitochondrial hormesis and diabetic complications. Diabetes 64, 663–672 (2015).
Sharma, K. et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 24, 1901–1912 (2013).
Kang, H.M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Dugan, L.L. et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Invest. 123, 4888–4899 (2013).
Susztak, K. Understanding the epigenetic syntax for the genetic alphabet in the kidney. J. Am. Soc. Nephrol. 25, 10–17 (2014).
Lunt, S.Y. et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol. Cell 57, 95–107 (2015).
Dayton, T.L. et al. Germline loss of PKM2 promotes metabolic distress and hepatocellular carcinoma. Genes Dev. 30, 1020–1033 (2016).
Luo, W. et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 (2011).
Shirai, T. et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 213, 337–354 (2016).
Yang, W. & Lu, Z. Nuclear PKM2 regulates the Warburg effect. Cell Cycle 12, 3154–3158 (2013).
Yang, W. et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature 480, 118–122 (2011).
Christofk, H.R., Vander Heiden, M.G., Wu, N., Asara, J.M. & Cantley, L.C. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452, 181–186 (2008).
Hitosugi, T. et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2, ra73 (2009).
Chaneton, B. et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491, 458–462 (2012).
Breyer, M.D. et al. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 16, 27–45 (2005).
Brosius, F.C. III et al. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 20, 2503–2512 (2009).
Qi, Z. et al. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54, 2628–2637 (2005).
Takahashi, T. & Harris, R.C. Role of endothelial nitric oxide synthase in diabetic nephropathy: lessons from diabetic eNOS knockout mice. J. Diabetes Res. 2014, 590541 (2014).
Gao, B.B., Stuart, L. & Feener, E.P. Label-free quantitative analysis of one-dimensional PAGE LC/MS/MS proteome: application on angiotensin II-stimulated smooth muscle cells secretome. Mol. Cell. Proteomics 7, 2399–2409 (2008).
Niewczas, M.A. et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 23, 507–515 (2012).
Sakairi, T. et al. Conditionally immortalized human podocyte cell lines established from urine. Am. J. Physiol. Renal Physiol. 298, F557–F567 (2010).
Shankland, S.J., Pippin, J.W., Reiser, J. & Mundel, P. Podocytes in culture: past, present, and future. Kidney Int. 72, 26–36 (2007).
Coppey, L.J. et al. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes 50, 1927–1937 (2001).
Li, B. et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 513, 251–255 (2014).
Inoguchi, T. et al. Insulin's effect on protein kinase C and diacylglycerol induced by diabetes and glucose in vascular tissues. Am. J. Physiol. 267, E369–E379 (1994).
Massart, J., Zierath, J.R. & Chibalin, A.V. A simple and rapid method to characterize lipid fate in skeletal muscle. BMC Res. Notes 7, 391 (2014).
Brown, J.M. et al. Isomer-specific regulation of metabolism and PPAR signaling by CLA in human preadipocytes. J. Lipid Res. 44, 1287–1300 (2003).
Nemet, I., Varga-Defterdarovic´, L. & Turk, Z. Preparation and quantification of methylglyoxal in human plasma using reverse-phase high-performance liquid chromatography. Clin. Biochem. 37, 875–881 (2004).
Sweetwyne, M.T. et al. Notch1 and Notch2 in podocytes play differential roles during diabetic nephropathy development. Diabetes 64, 4099–4111 (2015).
Takemoto, M. et al. A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol. 161, 799–805 (2002).
Advani, A. et al. Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J. Am. Soc. Nephrol. 20, 730–741 (2009).
Isermann, B. et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 13, 1349–1358 (2007).
Ma, L.J. et al. Divergent effects of low versus high dose anti-TGF-β antibody in puromycin aminonucleoside nephropathy in rats. Kidney Int. 65, 106–115 (2004).
Zhao, H.J. et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J. Am. Soc. Nephrol. 17, 2664–2669 (2006).
Pisania, A. et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab. Invest. 90, 1661–1675 (2010).
Acknowledgements
We are grateful to all the fellows who participated in Medalist Kidneys collection, D. Anastasiou, J. Lee, C. Thomas and M. Lauterbach for their technical advice. We also thank the technical support from E. Yu, daily-oral-gavaging team members, Z. Zsengeller and E. Pernicone. We are grateful to M. Maurer, who gave us technical advice on human kidney pathology assessment. We thank C. Clish for plasma metabolomics analysis. We are grateful to J. Hourihan for technical advice and review of the manuscript. W. Qi was a recipient of awards from the Juvenile Diabetes Research Foundation and Mary Iacocca Research Foundation. Mouse podocytes and human podocytes cell lines were kind gifts from P.M. and J.K. This project was funded by NIH 1DP3DK112192-01 (G.L.K.); DK094333-01 (G.L.K. and H.A.K.), 1R24DK090961-01 (G.L.K.); DRC grant-P30DK036836-30 (G.L.K.); JDRF 8-2005-358 (G.L.K.); JDRF 18-2008-363 (G.L.K.); JDRF17-2011-47 (G.L.K.); and JDRF17-2013-310 (H.A.K.). Additional research support was provided by the Thomas Beatson, Jr Foundation, the Brehm Foundation and a research grant from Sanofi-Aventis (G.L.K.). Other co-authors were supported by the following funding: DK107339-02 (M.A.Y.); Harold Whitworth Pierce Charitable Trust Postdoctoral Fellowship (A.M.B.); R01 DK109015 and R00 DK090210 (C.W.L.) and JDRF CDA-2015-89-A-B (M.A.N.).
Author information
Authors and Affiliations
Contributions
W.Q. designed and performed the experiments, analyzed the data and wrote the manuscript. H.A.K. assisted in the design of the Medalist study. Q.L., A.I., A.K., T.S., M.A.Y., S.L., L.J.C., A.P., C.W.L., G.Q., A.M.B. and W.J.I. provided technical support and/or critical discussions of the manuscript. I.-H.W. analyzed proteomics data. S.H. managed clinical data and samples. D.P. and L.T. performed all the clinical and some nonclinical statistical analysis and checked all the statistical analysis. C.C. performed electron-microscopy-related imaging work. M.A.N. analyzed metabolomics data. E.P.F. participated in proteomics analysis and critical review and discussion of the manuscript. M.G.V.H. provided technical advice on both TEPP-46 and Pkm2fl/fl mouse studies and constructs of pLHCX-Flag-mPKM2 and pLHCXFlag-mPKM1. I.E.S. and P.S.A. assessed all the human kidney pathology. R.C.S. provided critical review of the clinical data. G.L.K. supervised the project and wrote the manuscript. All the authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.K., T.S. and A.P. are employees of Sanofi-Aventis.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12; Supplementary Figures 13–21 (Uncropped Western Blots); Supplementary Tables 1–14 (PDF 3853 kb)
Dataset
Raw Data (XLSX 260 kb)
Supplementary Methods
Supplementary Methods (PDF 265 kb)
Rights and permissions
About this article
Cite this article
Qi, W., Keenan, H., Li, Q. et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med 23, 753–762 (2017). https://doi.org/10.1038/nm.4328
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4328
This article is cited by
-
Renal aging and mitochondrial quality control
Biogerontology (2024)
-
PGC1-α in diabetic kidney disease: unraveling renoprotection and molecular mechanisms
Molecular Biology Reports (2024)
-
Search progress of pyruvate kinase M2 (PKM2) in organ fibrosis
Molecular Biology Reports (2024)
-
M-type pyruvate kinase 2 (PKM2) tetramerization alleviates the progression of right ventricle failure by regulating oxidative stress and mitochondrial dynamics
Journal of Translational Medicine (2023)
-
BaoShenTongLuo formula protects against podocyte injury by regulating AMPK-mediated mitochondrial biogenesis in diabetic kidney disease
Chinese Medicine (2023)