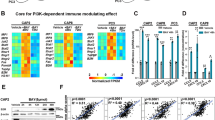
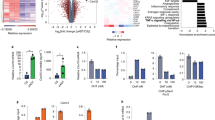
Abstract
To date, anti-CTLA-4 (ipilimumab) or anti-PD-1 (nivolumab) monotherapy has not been demonstrated to be of substantial clinical benefit in patients with prostate cancer. To identify additional immune-inhibitory pathways in the prostate-tumor microenvironment, we evaluated untreated and ipilimumab-treated tumors from patients in a presurgical clinical trial. Levels of the PD-L1 and VISTA inhibitory molecules increased on independent subsets of macrophages in treated tumors. Our data suggest that VISTA represents another compensatory inhibitory pathway in prostate tumors after ipilimumab therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Sharma, P. & Allison, J.P. Cell 161, 205–214 (2015).
Sharma, P. & Allison, J.P. Science 348, 56–61 (2015).
Kwon, E.D. et al. Lancet Oncol. 15, 700–712 (2014).
Topalian, S.L. et al. N. Engl. J. Med. 366, 2443–2454 (2012).
Subudhi, S.K. et al. Proc. Natl. Acad. Sci. USA 113, 11919–11924 (2016).
Liakou, C.I. et al. Proc. Natl. Acad. Sci. USA 105, 14987–14992 (2008).
Ng Tang, D. et al. Cancer Immunol. Res. 1, 229–234 (2013).
Carthon, B.C. et al. Clin. Cancer Res. 16, 2861–2871 (2010).
Freeman, G.J. et al. J. Exp. Med. 192, 1027–1034 (2000).
Lines, J.L., Sempere, L.F., Broughton, T., Wang, L. & Noelle, R. Cancer Immunol. Res. 2, 510–517 (2014).
Chen, H. et al. Proc. Natl. Acad. Sci. USA 106, 2729–2734 (2009).
Biswas, S.K. & Mantovani, A. Nat. Immunol. 11, 889–896 (2010).
Gao, J. et al. Oncogene 34, 5411–5417 (2015).
Wimberly, H. et al. Cancer Immunol. Res. 3, 326–332 (2015).
Stack, E.C., Wang, C., Roman, K.A. & Hoyt, C.C. Methods 70, 46–58 (2014).
Amir, el-A.D. et al. Nat. Biotechnol. 31, 545–552 (2013).
Levine, J.H. et al. Cell 162, 184–197 (2015).
Foster, B.A., Gingrich, J.R., Kwon, E.D., Madias, C. & Greenberg, N.M. Cancer Res. 57, 3325–3330 (1997).
Shi, L.Z. et al. Nat. Commun. 7, 12335 (2016).
Chen, P.L. et al. Cancer Discov. 6, 827–837 (2016).
Acknowledgements
We thank the clinical and research team for this study, including P. Corn, J. Araujo, B. Chapin, S. Matin, A. Zurita-Saavedra, J. Davis, L. Pisters, and A. Aparicio for treatment and care of patients. We thank M. Campbell for assistance with clinical data collection; L. Xiong, B. Guan, J. Kim, M. Higa, J. Bustillos, D. Ng Tang, and S. Basu for technical support; M. Curran and M. Ai for help with the TRAMP-C2 mouse models; Janssen, CA, for providing the anti-VISTA antibody; and C.G. Liu of the Sequencing & Non-Coding RNA Program for microarray studies. The clinical trial was supported by Bristol-Myers-Squibb (BMS). The research work was also supported by funding from the American Association for Cancer Research (AACR) Stand Up To Cancer–Cancer Research Institute Cancer Immunology Dream Team Translational Research Grant (SU2C-AACR-DT1012; J.P.A., P.S., S.K.S., J.G.); the Prostate Cancer Foundation (PCF) Challenge Grant in Immunology (J.P.A., P.S.); a PCF 2014 Young Investigator Award (S.K.S.); a Conquer Cancer Foundation–American Society of Clinical Oncology (ASCO) 2012 Young Investigator Award (J.G.); Cancer Prevention Research in Texas (CPRIT) RP120108 (P.S.); NIH/NCI R01 CA1633793 (P.S.); and NIH/NCI K12 CA088084 (J.G.) and NIH/NCI P30CA016672 (MD Anderson Cancer Center institutional grant); NIH/NCI P50 CA140388 Prostate Cancer SPORE (P.S., J.P.A., S.K.S., C.J.L., E.E.).
Author information
Authors and Affiliations
Contributions
P.S. designed the study; L.Z.S., J.B., L.M.V., J.C., I.I.W., M.A.S., J.S., and H.C. performed the experiments; J.G., J.F.W., C.A.P., L.Z.S., J.C., J.B., L.M.V., I.I.W., J.S., H.Z., J.W., E.E., and P.T. analyzed the data; J.G., P.S., J.P.A., S.K.S., J.W., and C.J.L. drafted the manuscript; and all authors reviewed the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.S. and J.P.A. are founders and advisors for Jounce Therapeutics. P.S. and J.P.A. are members of the Parker Institute for Cancer Immunotherapy. P.S. also serves as a consultant for BMS, AstraZeneca, Amgen, and GlaxoSmithKline. J.P.A. is an inventor of intellectual property owned by the University of California, Berkeley, and licensed to BMS and has received royalties from BMS. J.P.A. is also inventor of intellectual property owned by Memorial Sloan Kettering Cancer Center and licensed to Merck. J.G. serves as a consultant for Genentech. I.I.W. serves as a consultant for BMS. E.E. serves as a consultant for Janssen, Bayer, Medivation, Astellas, and Sanofi Takeda.
Supplementary information
Supplementary Text, Figures and Tables
Supplementary Discussion, Supplementary Figures 1–10 and Supplementary Tables 1–4 (PDF 15210 kb)
Source data
Rights and permissions
About this article
Cite this article
Gao, J., Ward, J., Pettaway, C. et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 23, 551–555 (2017). https://doi.org/10.1038/nm.4308
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4308
This article is cited by
-
Efficacy and safety of bispecific antibodies vs. immune checkpoint blockade combination therapy in cancer: a real-world comparison
Molecular Cancer (2024)
-
VISTA checkpoint inhibition by pH-selective antibody SNS-101 with optimized safety and pharmacokinetic profiles enhances PD-1 response
Nature Communications (2024)
-
Remodeling the tumor-immune microenvironment by anti-CTLA4 blockade enhanced subsequent anti-PD-1 efficacy in advanced nasopharyngeal carcinoma
npj Precision Oncology (2024)
-
Repositioning baloxavir marboxil as VISTA agonist that ameliorates experimental asthma
Cell Biology and Toxicology (2024)
-
Surgical Management and Considerations for Patients with Localized High-Risk Prostate Cancer
Current Treatment Options in Oncology (2024)