Abstract
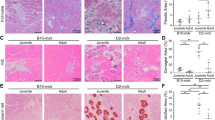
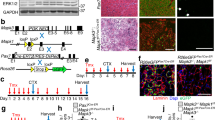
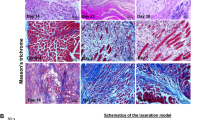
Interactions between stem cells and their microenvironment, or niche, are essential for stem cell maintenance and function. Our knowledge of the niche for the skeletal muscle stem cell, i.e., the satellite cell (SC), is incomplete. Here we show that β1-integrin is an essential niche molecule that maintains SC homeostasis, and sustains the expansion and self-renewal of this stem cell pool during regeneration. We further show that β1-integrin cooperates with fibroblast growth factor 2 (Fgf2), a potent growth factor for SCs, to synergistically activate their common downstream effectors, the mitogen-activated protein (MAP) kinase Erk and protein kinase B (Akt). Notably, SCs in aged mice show altered β1-integrin activity and insensitivity to Fgf2. Augmenting β1-integrin activity with a monoclonal antibody restores Fgf2 sensitivity and improves regeneration after experimentally induced muscle injury. The same treatment also enhances regeneration and function of dystrophic muscles in mdx mice, a model for Duchenne muscular dystrophy. Therefore, β1-integrin senses the SC niche to maintain responsiveness to Fgf2, and this integrin represents a potential therapeutic target for pathological conditions of the muscle in which the stem cell niche is compromised.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Ryall, J.G., Schertzer, J.D. & Lynch, G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 9, 213–228 (2008).
Chakkalakal, J.V., Jones, K.M., Basson, M.A. & Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 490, 355–360 (2012).
Fry, C.S. et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 21, 76–80 (2015).
Brack, A.S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Cosgrove, B.D. et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264 (2014).
Conboy, I.M., Conboy, M.J., Smythe, G.M. & Rando, T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577 (2003).
Shea, K.L. et al. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell 6, 117–129 (2010).
Carlson, M.E., Hsu, M. & Conboy, I.M. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature 454, 528–532 (2008).
Bernet, J.D. et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 20, 265–271 (2014).
Price, F.D. et al. Inhibition of JAK–STAT signaling stimulates adult satellite cell function. Nat. Med. 20, 1174–1181 (2014).
Tierney, M.T. et al. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 20, 1182–1186 (2014).
Mori, S. & Takada, Y. Cross-talk between fibroblast growth factor (FGF) receptor and integrin through direct integrin binding to FGF and resulting integrin–FGF–FGFR ternary complex formation. Med. Sci.(Basel) 1, 20–36 (2013).
Assoian, R.K. & Schwartz, M.A. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11, 48–53 (2001).
Hynes, R.O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Helbling-Leclerc, A. et al. Mutations in the laminin-α2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat. Genet. 11, 216–218 (1995).
Schwander, M. et al. β1-integrins regulate myoblast fusion and sarcomere assembly. Dev. Cell 4, 673–685 (2003).
Bulfield, G., Siller, W.G., Wight, P.A. & Moore, K.J. X-chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA 81, 1189–1192 (1984).
Lepper, C., Conway, S.J. & Fan, C.-M. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460, 627–631 (2009).
Raghavan, S., Bauer, C., Mundschau, G., Li, Q. & Fuchs, E. Conditional ablation of β1-integrin in skin. Severe defects in epidermal proliferation, basement membrane formation and hair follicle invagination. J. Cell Biol. 150, 1149–1160 (2000).
Webster, M.T. & Fan, C.-M. c-MET regulates myoblast motility and myocyte fusion during adult skeletal muscle regeneration. PLoS One 8, e81757 (2013).
Roovers, K., Davey, G., Zhu, X., Bottazzi, M.E. & Assoian, R.K. α5β1-integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth-factor-treated cells. Mol. Biol. Cell 10, 3197–3204 (1999).
Polanska, U.M., Fernig, D.G. & Kinnunen, T. Extracellular interactome of the FGF receptor–ligand system: complexities and the relative simplicity of the worm. Dev. Dyn. 238, 277–293 (2009).
Murakami, M., Elfenbein, A. & Simons, M. Noncanonical fibroblast growth factor signaling in angiogenesis. Cardiovasc. Res. 78, 223–231 (2008).
Miyamoto, S., Teramoto, H., Gutkind, J.S. & Yamada, K.M. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135, 1633–1642 (1996).
Jones, N.C., Fedorov, Y.V., Rosenthal, R.S. & Olwin, B.B. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. J. Cell. Physiol. 186, 104–115 (2001).
Siegel, A.L., Atchison, K., Fisher, K.E., Davis, G.E. & Cornelison, D.D. 3D time-lapse analysis of muscle satellite cell motility. Stem Cells 27, 2527–2538 (2009).
Troy, A. et al. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38-α/β MAPK. Cell Stem Cell 11, 541–553 (2012).
Conboy, I.M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Kragstrup, T.W., Kjaer, M. & Mackey, A.L. Structural, biochemical, cellular and functional changes in skeletal muscle extracellular matrix with aging. Scand. J. Med. Sci. Sports 21, 749–757 (2011).
Wood, L.K. et al. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J. Appl. Physiol. 117, 363–369 (2014).
Bazzoni, G., Shih, D.-T., Buck, C.A. & Hemler, M.E. Monoclonal antibody 9EG7 defines a novel β1-integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J. Biol. Chem. 270, 25570–25577 (1995).
Takagi, J. & Springer, T.A. Integrin activation and structural rearrangement. Immunol. Rev. 186, 141–163 (2002).
Calderwood, D.A., Shattil, S.J. & Ginsberg, M.H. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 275, 22607–22610 (2000).
Hintermann, E., Bilban, M., Sharabi, A. & Quaranta, V. Inhibitory role of α6β4-associated erbB-2 and phosphoinositide 3-kinase in keratinocyte haptotactic migration dependent on α3β1 integrin. J. Cell Biol. 153, 465–478 (2001).
Toledo, M.S., Suzuki, E., Handa, K. & Hakomori, S. Effect of ganglioside and tetraspanins in microdomains on interaction of integrins with fibroblast growth factor receptor. J. Biol. Chem. 280, 16227–16234 (2005).
Sahni, A. & Francis, C.W. Stimulation of endothelial cell proliferation by FGF2 in the presence of fibrinogen requires αvβ3. Blood 104, 3635–3641 (2004).
Lukjanenko et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med. http://dx.doi.org/10.1038/nm.4126 (2016).
Sicari, B.M. et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans, with volumetric muscle loss. Sci. Transl. Med. 6, 234ra58 (2014).
Fukada, S. et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25, 2448–2459 (2007).
Tanentzapf, G., Devenport, D., Godt, D. & Brown, N.H. Integrin-dependent anchoring of a stem cell niche. Nat. Cell Biol. 9, 1413–1418 (2007).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 (1999).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Hogan, B., Beddington, R., Costantini, F. & Lacy, E. Manipulating the Mouse Embryo: a Laboratory Manual (Cold Spring Harbor Laboratory Press, 1994).
Hakim, C.H., Wasala, N.B. & Duan, D. Evaluation of muscle function of the extensor digitorum longus muscle ex vivo and tibialis anterior muscle in situ in mice. J. Vis. Exp. 21, e50183 (2013).
Barton, E.R., Lynch, G. & Khurana, T.S. Measuring Isometric Force of Isolated Mouse Muscles In Vitro (TREAT–NMD Neuromuscular Network, 2008).
Trapnell, C. et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31, 46–53 (2013).
Acknowledgements
We thank E. Dikovskaia and S. Satchell for technical assistance, and C. Lepper, Y. Zheng and members of the Fan laboratory for comments. We especially thank A. Wagers for generously sharing the FACS and SC isolation protocol. Funding for this work was provided by the Carnegie Institution (C.-M.F.) and the National Institutes of Health (NIH) (grant no. AR060042; C.-M.F.). M.R. is supported by a predoctoral fellowship from the NIH (HD075345), and L.L. is supported by Carnegie Institution of Washington internal funds.
Author information
Authors and Affiliations
Contributions
M.R. and C.-M.F. conceptualized the study; M.R. performed experimental analysis for the mutant mice, as well as for the FACS and RNA-seq data, and demonstrated the utility of TS2/16; L.L. performed mechanistic experiments to investigate integrin–FGF synergy and conducted in situ muscle force measurements. C.-M.F. initiated and supervised the project. All three authors wrote, discussed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 44013 kb)
Rights and permissions
About this article
Cite this article
Rozo, M., Li, L. & Fan, CM. Targeting β1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat Med 22, 889–896 (2016). https://doi.org/10.1038/nm.4116
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4116
This article is cited by
-
Control of satellite cell function in muscle regeneration and its disruption in ageing
Nature Reviews Molecular Cell Biology (2022)
-
Molecular mechanisms of exercise contributing to tissue regeneration
Signal Transduction and Targeted Therapy (2022)
-
The jam session between muscle stem cells and the extracellular matrix in the tissue microenvironment
npj Regenerative Medicine (2022)
-
The landscape of aging
Science China Life Sciences (2022)
-
Analysis of human satellite cell dynamics on cultured adult skeletal muscle myofibers
Skeletal Muscle (2021)