Abstract
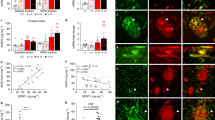
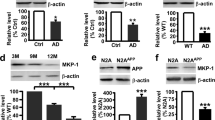
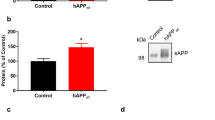
An increase in amyloid-β (Aβ) production is a major pathogenic mechanism associated with Alzheimer's disease (AD)1,2, but little is known about possible homeostatic control of the amyloidogenic pathway. Here we report that the amyloid precursor protein (APP) intracellular domain (AICD) downregulates Wiskott-Aldrich syndrome protein (WASP)-family verprolin homologous protein 1 (WAVE1 or WASF1) as part of a negative feedback mechanism to limit Aβ production. The AICD binds to the Wasf1 promoter, negatively regulates its transcription and downregulates Wasf1 mRNA and protein expression in Neuro 2a (N2a) cells. WAVE1 interacts and colocalizes with APP in the Golgi apparatus. Experimentally reducing WAVE1 in N2a cells decreased the budding of APP-containing vesicles and reduced cell-surface APP, thereby reducing the production of Aβ. WAVE1 downregulation was observed in mouse models of AD. Reduction of Wasf1 gene expression dramatically reduced Aβ levels and restored memory deficits in a mouse model of AD. A decrease in amounts of WASF1 mRNA was also observed in human AD brains, suggesting clinical relevance of the negative feedback circuit involved in homeostatic regulation of Aβ production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tanzi, R.E. & Bertram, L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell 120, 545–555 (2005).
Ballard, C. et al. Alzheimer's disease. Lancet 377, 1019–1031 (2011).
Takenawa, T. & Suetsugu, S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37–48 (2007).
Soderling, S.H. et al. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc. Natl. Acad. Sci. USA 100, 1723–1728 (2003).
Eden, S., Rohatgi, R., Podtelejnikov, A.V., Mann, M. & Kirschner, M.W. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418, 790–793 (2002).
Kim, Y. et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 442, 814–817 (2006).
Suzuki, T. et al. Molecular cloning of a novel apoptosis-related gene, human Nap1 (NCKAP1), and its possible relation to Alzheimer disease. Genomics 63, 246–254 (2000).
Oddo, S. et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421 (2003).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Lo, A.C., Haass, C., Wagner, S.L., Teplow, D.B. & Sisodia, S.S. Metabolism of the “Swedish” amyloid precursor protein variant in Madin-Darby canine kidney cells. J. Biol. Chem. 269, 30966–30973 (1994).
Müller, T., Meyer, H.E., Egensperger, R. & Marcus, K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer's disease. Prog. Neurobiol. 85, 393–406 (2008).
Haass, C., Kaether, C., Thinakaran, G. & Sisodia, S. Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med. 2, a006270 (2012).
Cao, X. & Sudhof, T.C. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293, 115–120 (2001).
Goodger, Z.V. et al. Nuclear signaling by the APP intracellular domain occurs predominantly through the amyloidogenic processing pathway. J. Cell Sci. 122, 3703–3714 (2009).
Belyaev, N.D. et al. The transcriptionally active amyloid precursor protein (APP) intracellular domain is preferentially produced from the 695 isoform of APP in a β-secretase-dependent pathway. J. Biol. Chem. 285, 41443–41454 (2010).
Flammang, B. et al. Evidence that the amyloid-β protein precursor intracellular domain, AICD, derives from β-secretase-generated C-terminal fragment. J. Alzheimers Dis. 30, 145–153 (2012).
Pardossi-Piquard, R. & Checler, F. The physiology of the β-amyloid precursor protein intracellular domain AICD. J. Neurochem. 120 (suppl. 1), 109–124 (2012).
Belyaev, N.D., Nalivaeva, N.N., Makova, N.Z. & Turner, A.J. Neprilysin gene expression requires binding of the amyloid precursor protein intracellular domain to its promoter: implications for Alzheimer disease. EMBO Rep. 10, 94–100 (2009).
Jankowsky, J.L. et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet. 13, 159–170 (2004).
Rajendran, L. & Annaert, W. Membrane trafficking pathways in Alzheimer's disease. Traffic 13, 759–770 (2012).
Suetsugu, S. & Gautreau, A. Synergistic BAR-NPF interactions in actin-driven membrane remodeling. Trends Cell Biol. 22, 141–150 (2012).
Anitei, M. & Hoflack, B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat. Cell Biol. 14, 11–19 (2012).
Webster, J.A. et al. Genetic control of human brain transcript expression in Alzheimer disease. Am. J. Hum. Genet. 84, 445–458 (2009).
Ceglia, I., Kim, Y., Nairn, A.C. & Greengard, P. Signaling pathways controlling the phosphorylation state of WAVE1, a regulator of actin polymerization. J. Neurochem. 114, 182–190 (2010).
Kamenetz, F. et al. APP processing and synaptic function. Neuron 37, 925–937 (2003).
Lesné, S. et al. NMDA receptor activation inhibits α-secretase and promotes neuronal amyloid-β production. J. Neurosci. 25, 9367–9377 (2005).
Cirrito, J.R. et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron 48, 913–922 (2005).
Konietzko, U. AICD nuclear signaling and its possible contribution to Alzheimer's disease. Curr. Alzheimer Res. 9, 200–216 (2012).
Nalivaeva, N.N. & Turner, A.J. The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett. 587, 2046–2054 (2013).
Gasparini, L. et al. Stimulation of β-amyloid precursor protein trafficking by insulin reduces intraneuronal β-amyloid and requires mitogen-activated protein kinase signaling. J. Neurosci. 21, 2561–2570 (2001).
He, G. et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer's disease. Nature 467, 95–98 (2010).
Cai, D. et al. Presenilin-1 uses phospholipase D1 as a negative regulator of β-amyloid formation. Proc. Natl. Acad. Sci. USA 103, 1941–1946 (2006).
Cai, D. et al. Presenilin-1 regulates intracellular trafficking and cell surface delivery of β-amyloid precursor protein. J. Biol. Chem. 278, 3446–3454 (2003).
Suetsugu, S., Yamazaki, D., Kurisu, S. & Takenawa, T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev. Cell 5, 595–609 (2003).
Thinakaran, G. et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17, 181–190 (1996).
Buxbaum, J.D. et al. Processing of Alzheimer β/A4 amyloid precursor protein: modulation by agents that regulate protein phosphorylation. Proc. Natl. Acad. Sci. USA 87, 6003–6006 (1990).
Soderling, S.H. et al. The WRP component of the WAVE-1 complex attenuates Rac-mediated signalling. Nat. Cell Biol. 4, 970–975 (2002).
Thinakaran, G., Teplow, D.B., Siman, R., Greenberg, B. & Sisodia, S.S. Metabolism of the “Swedish” amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the “beta-secretase” site occurs in the Golgi apparatus. J. Biol. Chem. 271, 9390–9397 (1996).
Urmoneit, B., Turner, J. & Dyrks, T. Pulse-chase experiments revealed β-secretase cleavage from immature full-length amyloid precursor protein harboring the Swedish mutation. Implications for distinct pathways. J. Mol. Neurosci. 11, 141–150 (1998).
Gresack, J.E., Kerr, K.M. & Frick, K.M. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol. Learn. Mem. 88, 393–408 (2007).
Harburger, L.L., Lambert, T.J. & Frick, K.M. Age-dependent effects of environmental enrichment on spatial reference memory in male mice. Behav. Brain Res. 185, 43–48 (2007).
Acknowledgements
This work was supported by the US National Institutes of Health (grants AG009464 (to P.G.) and AG047270 (to A.C.N.)), the Fisher Center for Alzheimer's Research Foundation (to P.G.) and the US Department of Defense–USAMRAA (grants W81XWH-09-1-0392 (to Y.K.) and W81XWH-09-1-0402 (to P.G.)). J.-H.A. was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2010-0027945). We thank W. Luo for protocols for metabolic pulse labeling and chase and organelle fractionation; W.J. Netzer (The Rockefeller University, New York, New York, USA) for sAPPβ antibody and mNotchΔE plasmid; D. Cai for the budding-assay protocol; and S.S. Sisodia for the pCB6-APP695wt and pCB6-APP695swe plasmids. We also thank A. Schaeffer, P. Kurup, P. Lombroso, D. Tampellini, G. Gouras and R. Moir for discussion or sharing of materials. We acknowledge R. Norinsky and The Rockefeller University Transgenics Services Laboratory for their excellent in vitro fertilization services, Z. Dong for research assistance and E. Griggs for graphics.
Author information
Authors and Affiliations
Contributions
Y.K., I.C., A.C.N. and P.G. designed experiments and wrote the manuscript. I.C. and Y.K. analyzed WAVE1 expression in N2a cells and mice. I.C. carried out ChIP and immunohistochemistry assays of WAVE1. J.-H.A. constructed plasmids including AICD-3×Flag and performed real-time PCR. I.C. and J.-H.A. performed promoter-luciferase assays. V.B. and I.C. analyzed AICD and Aβ expression. Y.K. and I.C. performed metabolic pulse labeling and chase experiments and in vitro budding assays. C.R. analyzed WAVE1 expression in human samples. X.Z. prepared mice. J.G. performed behavioral experiments. G.M., M.B., S.M.S. and Y.K. performed immunocytochemical experiments and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ceglia, I., Reitz, C., Gresack, J. et al. APP intracellular domain–WAVE1 pathway reduces amyloid-β production. Nat Med 21, 1054–1059 (2015). https://doi.org/10.1038/nm.3924
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3924
This article is cited by
-
A CHCHD6–APP axis connects amyloid and mitochondrial pathology in Alzheimer’s disease
Acta Neuropathologica (2022)
-
The nuclear transportation routes of membrane-bound transcription factors
Cell Communication and Signaling (2018)