Abstract
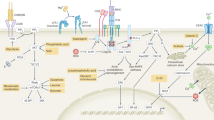
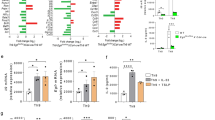
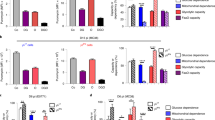
Our understanding of the pathways that regulate lymphocyte metabolism, as well as the effects of metabolism and its products on the immune response, is still limited. We report that a metabolic program controlled by the transcription factors hypoxia inducible factor-1α (HIF1-α) and aryl hydrocarbon receptor (AHR) supports the differentiation of type 1 regulatory T cell (Tr1) cells. HIF1-α controls the early metabolic reprograming of Tr1 cells. At later time points, AHR promotes HIF1-α degradation and takes control of Tr1 cell metabolism. Extracellular ATP (eATP) and hypoxia, linked to inflammation, trigger AHR inactivation by HIF1-α and inhibit Tr1 cell differentiation. Conversely, CD39 promotes Tr1 cell differentiation by depleting eATP. CD39 also contributes to Tr1 suppressive activity by generating adenosine in cooperation with CD73 expressed by responder T cells and antigen-presenting cells. These results suggest that HIF1-α and AHR integrate immunological, metabolic and environmental signals to regulate the immune response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ganeshan, K. & Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 32, 609–634 (2014).
MacIver, N.J., Michalek, R.D. & Rathmell, J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283 (2013).
Pearce, E.L., Poffenberger, M.C., Chang, C.H. & Jones, R.G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
Pollizzi, K.N. & Powell, J.D. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat. Rev. Immunol. 14, 435–446 (2014).
Powell, J.D., Pollizzi, K.N., Heikamp, E.B. & Horton, M.R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 30, 39–68 (2012).
Dang, E.V. et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Doedens, A.L. et al. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 14, 1173–1182 (2013).
Kim, J.S. et al. Natural and inducible TH17 cells are regulated differently by Akt and mTOR pathways. Nat. Immunol. 14, 611–618 (2013).
Michalek, R.D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Junger, W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 11, 201–212 (2011).
Quintana, F.J. & Sherr, D.H. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 65, 1148–1161 (2013).
Pot, C., Apetoh, L., Awasthi, A. & Kuchroo, V.K. Induction of regulatory Tr1 cells and inhibition of TH17 cells by IL-27. Semin. Immunol. 23, 438–445 (2011).
Roncarolo, M.G. et al. Interleukin-10–secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 212, 28–50 (2006).
Roncarolo, M.G., Gregori, S., Bacchetta, R. & Battaglia, M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr. Top. Microbiol. Immunol. 380, 39–68 (2014).
Awasthi, A. et al. A dominant function for interleukin 27 in generating interleukin 10–producing anti-inflammatory T cells. Nat. Immunol. 8, 1380–1389 (2007).
Stumhofer, J.S. et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8, 1363–1371 (2007).
Fitzgerald, D.C. et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27–stimulated T cells. Nat. Immunol. 8, 1372–1379 (2007).
Pot, C. et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10–producing Tr1 cells. J. Immunol. 183, 797–801 (2009).
Spolski, R., Kim, H.P., Zhu, W., Levy, D.E. & Leonard, W.J. IL-21 mediates suppressive effects via its induction of IL-10. J. Immunol. 182, 2859–2867 (2009).
Apetoh, L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 (2010).
Gandhi, R. et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell–like and Foxp3+ regulatory T cells. Nat. Immunol. 11, 846–853 (2010).
Eltzschig, H.K., Sitkovsky, M.V. & Robson, S.C. Purinergic signaling during inflammation. N. Engl. J. Med. 367, 2322–2333 (2012).
Sun, X. et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 139, 1030–1040 (2010).
Murugaiyan, G. et al. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J. Immunol. 183, 2435–2443 (2009).
Quintana, F.J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Wu, C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Mills, J.H. et al. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 105, 9325–9330 (2008).
Quintana, F.J. et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat. Immunol. 13, 770–777 (2012).
Gagliani, N. et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19, 739–746 (2013).
Kamanaka, M. et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knock-in tiger mouse. Immunity 25, 941–952 (2006).
Atarashi, K. et al. ATP drives lamina propria TH17 cell differentiation. Nature 455, 808–812 (2008).
Idzko, M., Ferrari, D. & Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 509, 310–317 (2014).
Mascanfroni, I.D. et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 14, 1054–1063 (2013).
Wang, C.M., Ploia, C., Anselmi, F., Sarukhan, A. & Viola, A. Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J. 33, 1354–1364 (2014).
Amoroso, F., Falzoni, S., Adinolfi, E., Ferrari, D. & Di Virgilio, F. The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis. 3, e370 (2012).
Sun, X. et al. Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/ENTPD1 null mice. Hepatology 57, 205–216 (2013).
Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012).
Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 9, 47–71 (2014).
Shi, L.Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Michalek, R.D. et al. Estrogen-related receptor-α is a metabolic regulator of effector T-cell activation and differentiation. Proc. Natl. Acad. Sci. USA 108, 18348–18353 (2011).
Eltzschig, H.K. & Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 (2011).
Saraiva, M. et al. Interleukin-10 production by TH1 cells requires interleukin-12–induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity 31, 209–219 (2009).
Chang, C.H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Ciofani, M. et al. A validated regulatory network for TH17 cell specification. Cell 151, 289–303 (2012).
Yosef, N. et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature 496, 461–468 (2013).
Enjyoji, K. et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 5, 1010–1017 (1999).
Yeste, A. et al. IL-21 induces IL-22 production in CD4+ T cells. Nat. Commun. 5, 3753 (2014).
Quintana, F.J. et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat. Immunol. 13, 770–777 (2012).
Wofford, J.A., Wieman, H.L., Jacobs, S.R., Zhao, Y. & Rathmell, J.C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111, 2101–2111 (2008).
Feng, L. et al. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia 13, 206–216 (2011).
Acknowledgements
We thank D. Frank (Dana-Farber Cancer Institute) for STAT3C plasmid. This work was supported by grants AI093903 and NS087867 from the US National Institutes of Health and RG4111A1 from the National Multiple Sclerosis Society to F.J.Q., and by grant CA164970 from the US National Institutes of Health to S.C.R. I.D.M. received support from an educational grant from Questcor (A219074) and by a postdoctoral fellowship (FG 2036-A1/1) from the National Multiple Sclerosis Society. M.C.T. is a graduate student in the Ph.D. program of the Federal University of São Paulo, and was supported by fellowship 246252/2012-0 from Ciências sem Fronteiras CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
Author information
Authors and Affiliations
Contributions
I.D.M., M.C.T., A.Y., Y.W., J.E.K. and C.B.C. performed in vitro and in vivo experiments; B.P. performed bioinformatics analysis; A.P. developed mathematical models; S.C.R., A.S.B., S.S., L.E.O., D.M.P. and F.P. provided unique reagents and discussed and/or interpreted findings; I.D.M. and F.J.Q. wrote the manuscript; and F.J.Q. designed and supervised the study and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 3161 kb)
Rights and permissions
About this article
Cite this article
Mascanfroni, I., Takenaka, M., Yeste, A. et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med 21, 638–646 (2015). https://doi.org/10.1038/nm.3868
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3868
This article is cited by
-
Uremic toxins mediate kidney diseases: the role of aryl hydrocarbon receptor
Cellular & Molecular Biology Letters (2024)
-
The P2X7 receptor in mucosal adaptive immunity
Purinergic Signalling (2024)
-
The metabolic potential of inflammatory and insulinaemic dietary patterns and risk of type 2 diabetes
Diabetologia (2024)
-
Modulating AHR function offers exciting therapeutic potential in gut immunity and inflammation
Cell & Bioscience (2023)
-
Review immune response of targeting CD39 in cancer
Biomarker Research (2023)