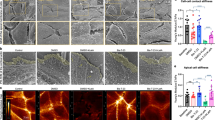
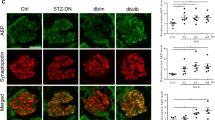
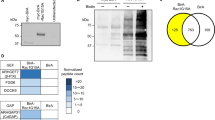
Abstract
Dysregulation of the actin cytoskeleton in podocytes represents a common pathway in the pathogenesis of proteinuria across a spectrum of chronic kidney diseases (CKD). The GTPase dynamin has been implicated in the maintenance of cellular architecture in podocytes through its direct interaction with actin. Furthermore, the propensity of dynamin to oligomerize into higher-order structures in an actin-dependent manner and to cross-link actin microfilaments into higher-order structures has been correlated with increased actin polymerization and global organization of the actin cytoskeleton in the cell. We found that use of the small molecule Bis-T-23, which promotes actin-dependent dynamin oligomerization and thus increased actin polymerization in injured podocytes, was sufficient to improve renal health in diverse models of both transient kidney disease and CKD. In particular, administration of Bis-T-23 in these renal disease models restored the normal ultrastructure of podocyte foot processes, lowered proteinuria, lowered collagen IV deposits in the mesangial matrix, diminished mesangial matrix expansion and extended lifespan. These results further establish that alterations in the actin cytoskeleton of kidney podocytes is a common hallmark of CKD, while also underscoring the substantial regenerative potential of injured glomeruli and identifying the oligomerization cycle of dynamin as an attractive potential therapeutic target to treat CKD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Meguid El Nahas, A. & Bello, A.K. Chronic kidney disease: the global challenge. Lancet 365, 331–340 (2005).
Saran, R., Hedgeman, E., Huseini, M., Stack, A. & Shahinian, V. Surveillance of chronic kidney disease around the world: tracking and reining in a global problem. Adv. Chronic Kidney Dis. 17, 271–281 (2010).
Haraldsson, B., Nystrom, J. & Deen, W.M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 88, 451–487 (2008).
Brown, E.J. et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 42, 72–76 (2010).
Boyer, O. et al. Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 22, 239–245 (2011).
Santín, S. et al. Nephrin mutations cause childhood- and adult-onset focal segmental glomerulosclerosis. Kidney Int. 76, 1268–1276 (2009).
Shih, N.Y. et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312–315 (1999).
Kaplan, J.M. et al. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Pagtalunan, M.E. et al. Podocyte loss and progressive glomerular injury in type II diabetes. J. Clin. Invest. 99, 342–348 (1997).
Faul, C., Asanuma, K., Yanagida-Asanuma, E., Kim, K. & Mundel, P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 (2007).
Wiggins, R.C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 71, 1205–1214 (2007).
Jefferson, J.A., Alpers, C.E. & Shankland, S.J. Podocyte biology for the bedside. Am. J. Kidney Dis. 58, 835–845 (2011).
Tryggvason, K., Patrakka, J. & Wartiovaara, J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N. Engl. J. Med. 354, 1387–1401 (2006).
Ronco, P. Proteinuria: is it all in the foot? J. Clin. Invest. 117, 2079–2082 (2007).
Seiler, M.W., Rennke, H.G., Venkatachalam, M.A. & Cotran, R.S. Pathogenesis of polycation-induced alterations (“fusion”) of glomerular epithelium. Lab. Invest. 36, 48–61 (1977).
Reiser, J. & Sever, S. Podocyte biology and pathogenesis of kidney disease. Annu. Rev. Med. 64, 357–366 (2013).
Sever, S. et al. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J. Clin. Invest. 117, 2095–2104 (2007).
Soda, K. et al. Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J. Clin. Invest. 122, 4401–4411 (2012).
Mettlen, M., Pucadyil, T., Ramachandran, R. & Schmid, S.L. Dissecting dynamin′s role in clathrin-mediated endocytosis. Biochem. Soc. Trans. 37, 1022–1026 (2009).
Sever, S., Chang, J. & Gu, C. Dynamin rings: not just for fission. Traffic 14, 1194–1199 (2013).
Hinshaw, J.E. Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 16, 483–519 (2000).
Gu, C. et al. Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J. 29, 3593–3606 (2010).
Ross, J.A. et al. Dimeric endophilin A2 stimulates assembly and GTPase activity of dynamin 2. Biophys. J. 100, 729–737 (2011).
Hill, T. et al. Small molecule inhibitors of dynamin I GTPase activity: development of dimeric tyrphostins. J. Med. Chem. 48, 7781–7788 (2005).
Gu, C. et al. Regulation of dynamin oligomerization in cells: the role of dynamin-actin interactions and its GTPase activity. Traffic 15, 819–838 (2014).
Gibbs, E.M. et al. Two dynamin-2 genes are required for normal zebrafish development. PLoS ONE 8, e55888 (2013).
Hanke, N. et al. “Zebrafishing” for novel genes relevant to the glomerular filtration barrier. BioMed Research International 2013, 658270 (2013).
Hentschel, D.M. et al. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am. J. Physiol. Renal Physiol. 293, F1746–F1750 (2007).
Kirsch, T. et al. Knockdown of the hypertension-associated gene NOSTRIN alters glomerular barrier function in zebrafish (Danio rerio). Hypertension 62, 726–730 (2013).
Song, B.D., Yarar, D. & Schmid, S.L. An assembly-incompetent mutant establishes a requirement for dynamin self-assembly in clathrin-mediated endocytosis in vivo. Mol. Biol. Cell 15, 2243–2252 (2004).
Faul, C. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 (2008).
Pippin, J.W. et al. Inducible rodent models of acquired podocyte diseases. Am. J. Physiol. Renal Physiol. 296, F213–F229 (2009).
Sever, S., Muhlberg, A.B. & Schmid, S.L. Impairment of dynamin′s GAP domain stimulates receptor-mediated endocytosis. Nature 398, 481–486 (1999).
Henderson, J.M., Al-Waheeb, S., Weins, A., Dandapani, S.V. & Pollak, M.R. Mice with altered α-actinin-4 expression have distinct morphologic patterns of glomerular disease. Kidney Int. 73, 741–750 (2008).
Yao, J. et al. α-Actinin-4-mediated FSGS: an inherited kidney disease caused by an aggregated and rapidly degraded cytoskeletal protein. PLoS Biol. 2, e167 (2004).
Tossidou, I. et al. CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J. Biol. Chem. 285, 25285–25295 (2010).
Meier, M. et al. Deletion of protein kinase C-ɛ signaling pathway induces glomerulosclerosis and tubulointerstitial fibrosis in vivo. J. Am. Soc. Nephrol. 18, 1190–1198 (2007).
Akita, Y. Protein kinase Cɛ: novel aspects of its multiple functions in cellular signaling. FEBS J. 275, 3987 (2008).
Chhabra, E.S. & Higgs, H.N. INF2 is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J. Biol. Chem. 281, 26754–26767 (2006).
Ruotsalainen, V. et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA 96, 7962–7967 (1999).
Graham, M.L., Janecek, J.L., Kittredge, J.A., Hering, B.J. & Schuurman, H.J. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp. Med. 61, 356–360 (2011).
Giganti, A. & Friederich, E. The actin cytoskeleton as a therapeutic target: state of the art and future directions. Prog. Cell Cycle Res. 5, 511–525 (2003).
Schiff, P.B., Fant, J. & Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature 277, 665–667 (1979).
Altschuler, Y. et al. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J. Cell Biol. 143, 1871–1881 (1998).
Gowrishankar, K. et al. Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell 149, 1353–1367 (2012).
Byron, A. et al. Glomerular cell cross-talk influences composition and assembly of extracellular matrix. J. Am. Soc. Nephrol. 25, 953–966 (2014).
Menon, M.C., Chuang, P.Y. & He, C.J. The glomerular filtration barrier: components and crosstalk. Int. J. Nephrol. 2012, 749010 (2012).
Shankland, S.J., Pippin, J.W. & Duffield, J.S. Progenitor cells and podocyte regeneration. Semin. Nephrol. 34, 418–428 (2014).
Daehn, I. et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J. Clin. Invest. 124, 1608–1621 (2014).
Quan, A. & Robinson, P.J. Rapid purification of native dynamin I and colorimetric GTPase assay. Methods Enzymol. 404, 556–569 (2005).
Leonard, M., Song, B.D., Ramachandran, R. & Schmid, S.L. Robust colorimetric assays for dynamin′s basal and stimulated GTPase activities. Methods Enzymol. 404, 490–503 (2005).
Mundel, P., Reiser, J. & Kriz, W. Induction of differentiation in cultured rat and human podocytes. J. Am. Soc. Nephrol. 8, 697–705 (1997).
Worthmann, K. et al. Def-6, a novel regulator of small GTPases in podocytes, acts downstream of atypical protein kinase C (aPKC) λ/ι. Am. J. Pathol. 183, 1945–1959 (2013).
Schiffer, M., Mundel, P., Shaw, A.S. & Bottinger, E.P. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-β-induced apoptosis. J. Biol. Chem. 279, 37004–37012 (2004).
Xie, J., Farage, E., Sugimoto, M. & Anand-Apte, B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev. Biol. 10, 76 (2010).
Ashworth, S. et al. Cofilin-1 inactivation leads to proteinuria–studies in zebrafish, mice and humans. PLoS ONE 5, e12626 (2010).
Hilfiker-Kleiner, D. et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128, 589–600 (2007).
Acknowledgements
This work was supported by the National Institutes of Health (R01 DK093773 and DK087985 to S.S.), the NephCure Foundation (S.S.), and Deutsche Forschungsgemeinschaft (SCHI587/3, 4, 6 to M.S. and REBIRTH 2 to D.H.-K. and S.E.). N.H. is the recipient of the New Investigator Award from Mount Desert Island Biological Laboratory. The DNA encoding genes for PKCɛ and mutant with impaired kinase activity were gifts from J.-W. Soh, Inha University, Incheon, South Korea. Immortalized podocytes from ACTN4 mice, ACTN4 mice and morpholino to downregulate inf2 were gifts from M. Pollak, Beth Israel Deaconess Medical Center, Boston, MA. Tg (L-FABP:DBP-EGFP) and Tg (l-fabp:DBP-EGFP) zebrafish were gifts from J. Xie and B. Anand-Apte, Cleveland Clinic, Cleveland, OH. PKCɛKO mice were gifts from M. Leitges, University of Oslo, Oslo, Norway. CD2APKO mice were gifts from A. Shaw, Washington University, St. Louis, MO.
Author information
Authors and Affiliations
Contributions
M.S., H.H., J.R. and S.S. designed the research; B.T., C.G., V.A.S., M.K., N.H., P.S., L.S., I.T., J.-K.P., S.E., D.H.-K., C.W., S.M., C.C., N.T., S.H., S.R., M.K.S., A.V. and F.G. performed the research. B.T., C.G., M.K., M.S., and S.S. analyzed the data. M.S., B.T., C.G. and S.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.S. and J.R. have pending or issued patents on novel kidney-protective therapies that have been out-licensed to Trisaq Inc. in which they have financial interest. In addition, they stand to gain royalties from their commercialization. The remaining authors report no conflicts.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 49110 kb)
Rights and permissions
About this article
Cite this article
Schiffer, M., Teng, B., Gu, C. et al. Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models. Nat Med 21, 601–609 (2015). https://doi.org/10.1038/nm.3843
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3843
This article is cited by
-
Urinary exosome proteins PAK6 and EGFR as noninvasive diagnostic biomarkers of diabetic nephropathy
BMC Nephrology (2023)
-
Simultaneous stabilization of actin cytoskeleton in multiple nephron-specific cells protects the kidney from diverse injury
Nature Communications (2022)
-
Slit diaphragm maintenance requires dynamic clathrin-mediated endocytosis facilitated by AP-2, Lap, Aux and Hsc70-4 in nephrocytes
Cell & Bioscience (2021)
-
The glomerular filtration barrier: a structural target for novel kidney therapies
Nature Reviews Drug Discovery (2021)
-
Role of actin cytoskeleton in podocytes
Pediatric Nephrology (2021)