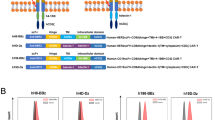
Abstract
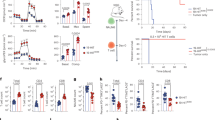
Chimeric antigen receptors (CARs) targeting CD19 have mediated dramatic antitumor responses in hematologic malignancies, but tumor regression has rarely occurred using CARs targeting other antigens. It remains unknown whether the impressive effects of CD19 CARs relate to greater susceptibility of hematologic malignancies to CAR therapies, or superior functionality of the CD19 CAR itself. We show that tonic CAR CD3-ζ phosphorylation, triggered by antigen-independent clustering of CAR single-chain variable fragments, can induce early exhaustion of CAR T cells that limits antitumor efficacy. Such activation is present to varying degrees in all CARs studied, except the highly effective CD19 CAR. We further determine that CD28 costimulation augments, whereas 4-1BB costimulation reduces, exhaustion induced by persistent CAR signaling. Our results provide biological explanations for the antitumor effects of CD19 CARs and for the observations that CD19 CAR T cells incorporating the 4-1BB costimulatory domain are more persistent than those incorporating CD28 in clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Referenced accessions
GenBank/EMBL/DDBJ
References
Lee, D.W. et al. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin. Cancer Res. 18, 2780–2790 (2012).
Sadelain, M., Brentjens, R. & Rivière, I. The basic principles of chimeric antigen receptor design. Cancer Discovery 3, 388–398 (2013).
Lee, D.W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Maude, S.L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Kochenderfer, J.N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood 119, 2709–2720 (2012).
Porter, D.L. et al. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Grupp, S.A. et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Savoldo, B. et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients. J. Clin. Invest. 121, 1822–1826 (2011).
Brentjens, R.J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra138 (2013).
Davila, M.L. et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra225 (2014).
Kershaw, M.H. et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 (2006).
Park, J.R. et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 15, 825–833 (2007).
Pule, M.A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
Louis, C.U. et al. Antitumor activity and long-term fate of chimeric antigen receptor–positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
Till, B.G. et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: pilot clinical trial results. Blood 119, 3940–3950 (2012).
Lamers, C.H.J. et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 21, 904–912 (2013).
Robbins, P.F. et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J. Immunol. 173, 7125–7130 (2004).
Kowolik, C.M. et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 66, 10995–11004 (2006).
Milone, M.C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Haso, W. et al. Anti-CD22–chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1174 (2013).
Ahmadzadeh, M. et al. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Baitsch, L. et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Zhou, Q. et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 117, 4501–4510 (2011).
Woo, S.-R. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012).
Topalian, S.L. et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Virgin, H.W., Wherry, E.J. & Ahmed, R. Redefining chronic viral infection. Cell 138, 30–50 (2009).
Wherry, E.J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Rossig, C. et al. Targeting of GD2-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int. J. Cancer 94, 228–236 (2001).
Pulè, M.A. et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 12, 933–941 (2005).
Hudecek, M. et al. The non-signaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol. Res. 3, 125–135 (2014).
Kochenderfer, J.N. et al. Construction and pre-clinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 32, 689–702 (2009).
Shin, H. et al. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity 31, 309–320 (2009).
Doering, T.A. et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144 (2012).
Paley, M.A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012).
Friend, L.D. et al. A dose-dependent requirement for the proline motif of CD28 in cellular and humoral immunity revealed by a targeted knockin mutant. J. Exp. Med. 203, 2121–2133 (2006).
Dodson, L.F. et al. Targeted knock-in mice expressing mutations of CD28 reveal an essential pathway for costimulation. Mol. Cell. Biol. 29, 3710–3721 (2009).
Zhao, Y. et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J. Immunol. 183, 5563–5574 (2009).
Nieba, L., Honegger, A., Krebber, C. & Plückthun, A. Disrupting the hydrophobic patches at the antibody variable/constant domain interface: improved in vivo folding and physical characterization of an engineered scFv fragment. Protein Eng. 10, 435–444 (1997).
Dolezal, O. et al. Single-chain Fv multimers of the anti-neuraminidase antibody NC10: the residue at position 15 in the VL domain of the scFv-0 (VL− VH) molecule is primarily responsible for formation of a tetramer–trimer equilibrium. Protein Eng. 16, 47–56 (2003).
Whitlow, M. et al. Multivalent Fvs: characterization of single-chain Fv oligomers and preparation of a bispecific Fv. Protein Eng. 7, 1017–1026 (1994).
Wherry, E.J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Crawford, A. et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Guest, R.D. et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J. Immunother. 28, 203–211 (2005).
James, S.E. et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J. Immunol. 180, 7028–7038 (2008).
Frigault, M.J. et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol. Res. http://dx.doi.org/10.1158/2326-6066.CIR-14-0186 (2015).
Agnellini, P. et al. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc. Natl. Acad. Sci. USA 104, 4565–4570 (2007).
Vezys, V. et al. 4–1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J. Immunol. 187, 1634–1642 (2011).
Wang, C. et al. 4–1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J. Immunol. 179, 8252–8263 (2007).
Dittus, C. et al. Chimeric antigen receptor modified T cells directed against CD19 (CTL019 cells) have long-term persistence and induce durable responses in relapsed, refractory CLL. Blood 122, 4162 (2013).
Doedens, A.L. et al. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 14, 1173–1182 (2013).
Pearce, E.L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
van der Windt, G.J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Roederer, M., Nozzi, J.L. & Nason, M.C. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79, 167–174 (2011).
Morgan, R.A. et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum. Gene Ther. 23, 1043–1053 (2012).
Brochet, X., Lefranc, M.-P. & Giudicelli, V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 36 (suppl. 2), W503–W508 (2008).
Sen, G. et al. Induction of IgG antibodies by an anti-idiotype antibody mimicking disialoganglioside GD2. J. Immunother. 21, 75–83 (1998).
Jena, B. et al. Chimeric antigen receptor (CAR)-specific monoclonal antibody to detect CD19-specific T cells in clinical trials. PLoS ONE 8, e57838 (2013).
Edelstein, A. et al. Computer control of microscopes using μManager. Curr. Protoc. Mol. Biol. 92, 14.20.1–14.20.17 (2010).
Elangovan, M. et al. Characterization of one-and two-photon excitation fluorescence resonance energy transfer microscopy. Methods 29, 58–73 (2003).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Olenych, S.G., Claxton, N.S., Ottenberg, G.K. & Davidson, M.W. The fluorescent protein color palette. Curr. Protoc. Cell Biol. 21, 21.5 (2007).
Martin, C.E. et al. IL-7/anti–IL-7 mAb complexes augment cytokine potency in mice through association with IgG-Fc and by competition with IL-7R. Blood 121, 4484–4492 (2013).
Acknowledgements
We kindly thank S. Gottschalk (Texas Children's Hospital) for providing sequences for the 14g2a scFv and IgG1 CH2CH3; J. Kochenderfer (National Cancer Institute, NCI, US National Institutes of Health, NIH) for the H3 MSGV-FMC63-28z retroviral vector producer line; S. Grupp (Children's Hospital of Philadelphia) for the NALM6-GL cell line; R. Morgan (BlueBird Bio, previously NCI, NIH) for the MSGV-139-28z and MSGV-4D5-28BBz vectors; and C. June (University of Pennsylvania) for sequences to the 4-1BB CAR endodomain. We also thank L. Cooper (M.D. Anderson) for providing the 136.20.1, FMC63 anti-idiotype antibody; the Biological Research Branch of NCI for providing the 1A7, 14g2a anti-idiotype antibody; the Clinical Support Laboratory of the Frederick National Laboratory for Cancer Research (FNLCR) for assisting in MesoScale cytokine release assays; and the Laboratory of Molecular Technology (LMT) Microarray Group of FNLCR for assisting in microarray assays. We thank N. Restifo and M. Roederer for careful review of this manuscript. This work was supported by the Intramural Research Program of the NIH, including the NCI and the National Institute of Biomedical Imaging and Bioengineering (NIBIB): ZIA BC 011073 (A.H.L., K.M.W., J.P.S., A.J.W., M.E.K., V.R.V., R.J.O. and C.L.M); ZIA BC 011565 (W.M.H. and T.J.F.); ZIA BC 011332 (M.M. and R.N.K.); and ZIA EB 000071-06 (M.I. and G.H.P.) This research was also supported by a Stand Up To Cancer – St. Baldrick's – NCI Pediatric Dream Team Translational Cancer Research Grant. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Author information
Authors and Affiliations
Contributions
A.H.L., W.M.H., J.F.S., M.M., M.I., M.E.K., R.N.K., G.H.P., T.J.F., R.J.O. and C.L.M. designed the research; A.H.L., W.M.H., K.M.W., M.M., M.I., J.P.S., A.J.W., M.E.K., V.R.V. and G.H.P. conducted experiments; A.H.L., W.M.H., J.F.S., M.M., M.I., G.H.P., T.J.F., R.J.O. and C.L.M. analyzed data; and A.H.L. and C.L.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
R.J.O. and C.L.M. are co-inventors of the CD22-CAR described in this report. The authors declare no other competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 (PDF 6526 kb)
Supplementary Table 1
All Probes - All comparisons (XLSX 24877 kb)
Rights and permissions
About this article
Cite this article
Long, A., Haso, W., Shern, J. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21, 581–590 (2015). https://doi.org/10.1038/nm.3838
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3838
This article is cited by
-
Tuning spacer length improves the functionality of the nanobody-based VEGFR2 CAR T cell
BMC Biotechnology (2024)
-
Hypothesis of a CD137/Eomes activating axis for effector T cells in HPV oropharyngeal cancers
Molecular Medicine (2024)
-
SMAD7 expression in CAR-T cells improves persistence and safety for solid tumors
Cellular & Molecular Immunology (2024)
-
IL-10-expressing CAR T cells resist dysfunction and mediate durable clearance of solid tumors and metastases
Nature Biotechnology (2024)
-
Chimeric antigen receptor-based natural killer cell immunotherapy in cancer: from bench to bedside
Cell Death & Disease (2024)