Abstract
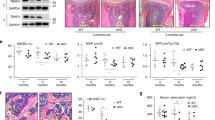
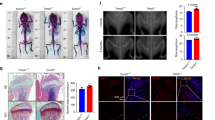
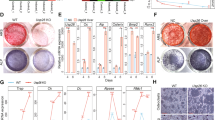
Aging-related bone loss and osteoporosis affect millions of people worldwide. Chronic inflammation associated with aging promotes bone resorption and impairs bone formation. Here we show that Wnt4 attenuates bone loss in osteoporosis and skeletal aging mouse models by inhibiting nuclear factor-κB (NF-κB) via noncanonical Wnt signaling. Transgenic mice expressing Wnt4 from osteoblasts were significantly protected from bone loss and chronic inflammation induced by ovariectomy, tumor necrosis factor or natural aging. In addition to promoting bone formation, Wnt4 inhibited osteoclast formation and bone resorption. Mechanistically, Wnt4 inhibited NF-κB activation mediated by transforming growth factor-β–activated kinase-1 (Tak1) in macrophages and osteoclast precursors independently of β-catenin. Moreover, recombinant Wnt4 alleviated bone loss and inflammation by inhibiting NF-κB in vivo in mouse models of bone disease. Given its dual role in promoting bone formation and inhibiting bone resorption, our results suggest that Wnt4 signaling could be an attractive therapeutic target for treating osteoporosis and preventing skeletal aging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
04 August 2015
In the published article, we, the authors, interpreted the data in Figure 4a–e to mean that transgenic expression of Wnt4 in osteoblasts of mice protects them from age-related bone loss. However, after publication it has been pointed out to us that, at all the ages examined, the transgenic mice had greater trabecular bone mass than control mice. Thus, we have re-examined the data in Figure 4b using statistical tests to examine the relative rate of change of bone mass and bone volume/total volume over the four age groups presented, and we find there is no statistical difference for the rate of these parameters between the transgenic and control groups. Thus, our conclusions with respect to this aspect of the study were incorrect, and further we conclude that we placed an improper emphasis on these findings in the title of the paper. We have also reanalyzed the data in the rest of the paper using more proper statistical tests in several instances. In particular, the standard deviations in Figures 1g, 1h, 2c–e, 3c–e, 4d, 4f, 6d, 6e and Supplementary Figure 6g were reported inappropriately. We used values from each histology image (3–6 images per mouse) as individual data points, instead of the mean values for each mouse, leading to an increased standard deviation. Furthermore, for morphometric and serum analysis in Figures 2, 3, 4g,h, 6 and Supplementary Figure 6, one-way analysis of variance with Tukey's post hoc test should have been used to account for multiple comparisons and adjustments for type I errors. Upon reanalysis, the comparison of osteoclast numbers/bone surface between the wild-type (WT) sham group and the Ob-Wnt4 sham group in Figure 2e, and the comparison of osteoclast surface/bone surface between the WT and Wnt4 groups in Figure 3e lost statistical significance, as was stated in the article. Although our conclusion about the effect of transgenic expression of Wnt4 in osteoblasts on skeletal aging appears to be incorrect, the above changes regarding our statistical analyses do not alter the conclusions drawn in the manuscript with respect to the effect of Wnt4 transgenic expression on bone mass compared to non-transgenic mice at static time points, the effect of recombinant Wnt4 on bone loss in the ovariectomy model nor on the molecular mechanisms for these effects. Nonetheless, we apologize for any confusion these original analyses or conclusions may have caused.
References
Manolagas, S.C. & Jilka, R.L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 332, 305–311 (1995).
Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 13, 791–801 (2007).
Manolagas, S.C. & Parfitt, A.M. What old means to bone. Trends Endocrinol. Metab. 21, 369–374 (2010).
Chien, K.R. & Karsenty, G. Longevity and lineages: toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell 120, 533–544 (2005).
Bruunsgaard, H. et al. Predicting death from tumour necrosis factor-α and interleukin-6 in 80-year-old people. Clin. Exp. Immunol. 132, 24–31 (2003).
Weitzmann, M.N. & Pacifici, R. Estrogen deficiency and bone loss: an inflammatory tale. J. Clin. Invest. 116, 1186–1194 (2006).
McLean, R.R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 7, 134–139 (2009).
López-Otín, C., Blasco, M.A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Teitelbaum, S.L. Bone resorption by osteoclasts. Science 289, 1504–1508 (2000).
Wagner, E.F. & Karsenty, G. Genetic control of skeletal development. Curr. Opin. Genet. Dev. 11, 527–532 (2001).
Raisz, L.G. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J. Clin. Invest. 115, 3318–3325 (2005).
Riggs, B.L., Khosla, S. & Melton, L.J. III. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 13, 763–773 (1998).
Khosla, S. & Riggs, B.L. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol. Metab. Clin. North Am. 34, 1015–1030 (2005).
Sun, L. et al. FSH directly regulates bone mass. Cell 125, 247–260 (2006).
Ghosh, S. & Karin, M. Missing pieces in the NF-κB puzzle. Cell 109, S81–S96 (2002).
Boyce, B.F., Yao, Z. & Xing, L. Functions of nuclear factor κB in bone. Ann. NY Acad. Sci. 1192, 367–375 (2010).
Jimi, E. & Ghosh, S. Role of nuclear factor-κB in the immune system and bone. Immunol. Rev. 208, 80–87 (2005).
Krum, S.A., Chang, J., Miranda-Carboni, G. & Wang, C.Y. Novel functions for NF-κB: inhibition of bone formation. Nat. Rev. Rheumatol. 6, 607–611 (2010).
Almeida, M., Han, L., Ambrogini, E., Bartell, S.M. & Manolagas, S.C. Oxidative stress stimulates apoptosis and activates NF-κB in osteoblastic cells via a PKCβ/p66shc signaling cascade: counter regulation by estrogens or androgens. Mol. Endocrinol. 24, 2030–2037 (2010).
Jimi, E. et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 10, 617–624 (2004).
Chen, Q. et al. DNA damage drives accelerated bone aging via an NF-κB–dependent mechanism. J. Bone Miner. Res. 28, 1214–1228 (2013).
Chang, J. et al. Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat. Med. 15, 682–689 (2009).
Anastas, J.N. & Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 (2013).
MacDonald, B.T., Tamai, K. & He, X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009).
Regard, J.B., Zhong, Z., Williams, B.O. & Yang, Y. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb. Perspect. Biol. 4, 1–12 (2012).
Baron, R. & Kneissel, M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 (2013).
Veeman, M.T., Axelrod, J.D. & Moon, R.T. A second canon. Functions and mechanisms of β-catenin–independent Wnt signaling. Dev. Cell 5, 367–377 (2003).
Seifert, J.R. & Mlodzik, M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126–138 (2007).
McNeill, H. & Woodgett, J.R. When pathways collide: collaboration and connivance among signalling proteins in development. Nat. Rev. Mol. Cell Biol. 11, 404–413 (2010).
Lako, M. et al. Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene 219, 101–110 (1998).
Qiu, W., Chen, L. & Kassem, M. Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells. Biochem. Biophys. Res. Commun. 413, 98–104 (2011).
Maeda, K. et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med. 18, 405–412 (2012).
Chang, J. et al. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J. Biol. Chem. 282, 30938–30948 (2007).
Krebsbach, P.H. et al. Transgenic expression of COL1A1-chloramphenicol acetyltransferase fusion genes in bone: differential utilization of promoter elements in vivo and in cultured cells. Mol. Cell. Biol. 13, 5168–5174 (1993).
Liu, F. et al. Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int. J. Dev. Biol. 48, 645–653 (2004).
Khosla, S., Westendorf, J.J. & Oursler, M.J. Building bone to reverse osteoporosis and repair fractures. J. Clin. Invest. 118, 421–428 (2008).
Halleen, J.M. et al. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J. Bone Miner. Res. 15, 1337–1345 (2000).
Pacifici, R. et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc. Natl. Acad. Sci. USA 88, 5134–5138 (1991).
Jilka, R.L. et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257, 88–91 (1992).
Hayward, M.D. et al. An extensive phenotypic characterization of the hTNFα transgenic mice. BMC Physiol. 7, 13 (2007).
Guo, R. et al. Ubiquitin ligase Smurf1 mediates tumor necrosis factor-induced systemic bone loss by promoting proteasomal degradation of bone morphogenetic signaling proteins. J. Biol. Chem. 283, 23084–23092 (2008).
Yao, Z., Xing, L. & Boyce, B.F. NF-κB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J. Clin. Invest. 119, 3024–3034 (2009).
Lam, J. et al. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 106, 1481–1488 (2000).
Pandey, A.C. et al. MicroRNA profiling reveals age-dependent differential expression of nuclear factor κB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res. Ther. 2, 49 (2011).
Garnero, P., Sornay-Rendu, E., Chapuy, M.C. & Delmas, P.D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Miner. Res. 11, 337–349 (1996).
Ginaldi, L., Di Benedetto, M.C. & De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2, 14 (2005).
Lomaga, M.A. et al. TRAF6 deficiency results in osteopetrosisa and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 (1999).
Bai, S., Zha, J., Zhao, H., Ross, F.P. & Teitelbaum, S.L. Tumor necrosis factor receptor-associated factor 6 is an intranuclear transcriptional coactivator in osteoclasts. J. Biol. Chem. 283, 30861–30867 (2008).
Mizukami, J. et al. Receptor activator of NF-κB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol. Cell. Biol. 22, 992–1000 (2002).
Takada, I. et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-γ transactivation. Nat. Cell Biol. 9, 1273–1285 (2007).
Teitelbaum, S.L. & Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 (2003).
Asagiri, M. et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 202, 1261–1269 (2005).
Holmen, S.L. et al. Essential role of β-catenin in postnatal bone acquisition. J. Biol. Chem. 280, 21162–21168 (2005).
Lories, R.J., Corr, M. & Lane, N.E. To Wnt or not to Wnt: the bone and joint health dilemma. Nat. Rev. Rheumatol. 9, 328–339 (2013).
Winkel, A. et al. Wnt-ligand–dependent interaction of TAK1 (TGF-β–activated kinase-1) with the receptor tyrosine kinase Ror2 modulates canonical Wnt-signalling. Cell. Signal. 20, 2134–2144 (2008).
Li, M. et al. TAB2 scaffolds TAK1 and NLK in repressing canonical Wnt signaling. J. Biol. Chem. 285, 13397–13404 (2010).
Sugimura, R. et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 150, 351–365 (2012).
Heinonen, K.M., Vanegas, J.R., Lew, D., Krosl, J. & Perreault, C. Wnt4 enhances murine hematopoietic progenitor cell expansion through a planar cell polarity-like pathway. PLoS ONE 6, e19279 (2011).
Fan, Z. et al. BCOR regulates mesenchymal stem cell function by epigenetic mechanisms. Nat. Cell Biol. 11, 1002–1009 (2009).
Redlich, K. et al. Osteoclasts are essential for TNF-α-mediated joint destruction. J. Clin. Invest. 110, 1419–1427 (2002).
Thwin, M.M. et al. Effect of phospholipase A2 inhibitory peptide on inflammatory arthritis in a TNF transgenic mouse model: a time-course ultrastructural study. Arthritis Res. Ther. 6, R282–R294 (2004).
Li, J. et al. LATS2 suppresses oncogenic Wnt signaling by disrupting β-catenin/BCL9 interaction. Cell Rep. 5, 1650–1663 (2013).
Acknowledgements
We thank J. Adams for valuable advice. This work was supported by the US National Institute of Dental and Craniofacial Research grants DE19412 and DE16513 (to C.-Y.W.), the US National Institute of Arthritis and Musculoskeletal and Skin Diseases grant AR63089 (to C.-Y.W.) and the UCLA Broad Stem Cell Research Center Research Award (to C.-Y.W.).
Author information
Authors and Affiliations
Contributions
B.Y., J.C., Y.L., J.L. and K.K. performed the experiments. K.A.-H., D.T.G., N.-H.P. and C.-Y.W. designed experiments and analyzed data. B.Y. and C.-Y.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 4831 kb)
Rights and permissions
About this article
Cite this article
Yu, B., Chang, J., Liu, Y. et al. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-κB. Nat Med 20, 1009–1017 (2014). https://doi.org/10.1038/nm.3586
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3586
This article is cited by
-
Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders
Osteoporosis International (2023)
-
Desferrioxamine alleviates UHMWPE particle-induced osteoclastic osteolysis by inhibiting caspase-1-dependent pyroptosis in osteocytes
Journal of Biological Engineering (2022)
-
Synthesis and anti-influenza virus activity evaluation of novel andrographolide derivatives
Medicinal Chemistry Research (2022)
-
SETD2-mediated epigenetic regulation of noncanonical Wnt5A during osteoclastogenesis
Clinical Epigenetics (2021)
-
Noncanonical Wnt5a signaling regulates tendon stem/progenitor cells senescence
Stem Cell Research & Therapy (2021)