Abstract
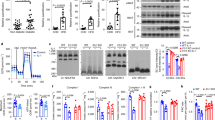
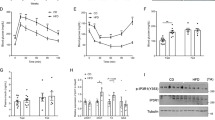
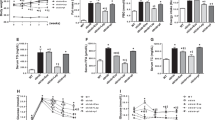
We previously demonstrated that low biosynthesis of ω–3 fatty acid–derived proresolution mediators, termed protectins, is associated with an impaired global resolution capacity, inflammation and insulin resistance in obese high-fat diet–fed mice1. These findings prompted a more direct study of the therapeutic potential of protectins for the treatment of metabolic disorders. Herein we show that protectin DX (PDX) exerts an unanticipated glucoregulatory activity that is distinct from its anti-inflammatory actions. We found that PDX selectively stimulated the release of the prototypic myokine interleukin-6 (IL-6) from skeletal muscle and thereby initiated a myokine-liver signaling axis, which blunted hepatic glucose production via signal transducer and activator of transcription 3 (STAT3)-mediated transcriptional suppression of the gluconeogenic program. These effects of PDX were abrogated in Il6-null mice. PDX also activated AMP-activated protein kinase (AMPK); however, it did so in an IL-6–independent manner. Notably, we demonstrated that administration of PDX to obese diabetic db/db mice raises skeletal muscle IL-6 levels and substantially improves their insulin sensitivity without any impact on adipose tissue inflammation. Our findings thus support the development of PDX-based selective muscle IL-6 secretagogues as a new class of therapy for the treatment of insulin resistance and type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
White, P.J., Arita, M., Taguchi, R., Kang, J.X. & Marette, A. Transgenic restoration of long-chain n–3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat–fed mice. Diabetes 59, 3066–3073 (2010).
Serhan, C.N. et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 176, 1848–1859 (2006).
Chen, P. et al. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 583, 3478–3484 (2009).
Awazawa, M. et al. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6–dependent pathway. Cell Metab. 13, 401–412 (2011).
Pedersen, B.K. & Febbraio, M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 (2008).
Pedersen, B.K. & Febbraio, M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Stanford, K.I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 (2013).
Sag, D., Carling, D., Stout, R.D. & Suttles, J. Adenosine 5′-monophosphate–activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 181, 8633–8641 (2008).
Inoue, H. et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 3, 267–275 (2006).
Inoue, H. et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat. Med. 10, 168–174 (2004).
Carey, A.L. et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55, 2688–2697 (2006).
Kelly, M., Gauthier, M.S., Saha, A.K. & Ruderman, N.B. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes 58, 1953–1960 (2009).
Kelly, M. et al. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem. Biophys. Res. Commun. 320, 449–454 (2004).
Clària, J., Dalli, J., Yacoubian, S., Gao, F. & Serhan, C.N. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J. Immunol. 189, 2597–2605 (2012).
Jové, M., Planavila, A., Laguna, J.C. & Vázquez-Carrera, M. Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor κB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology 146, 3087–3095 (2005).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Wunderlich, F.T. et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 12, 237–249 (2010).
Pedersen, B.K. & Febbraio, M.A. Point: interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 102, 814–816 (2007).
Mauer, J. et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 15, 423–430 (2014).
Gray, S.R., Ratkevicius, A., Wackerhage, H., Coats, P. & Nimmo, M.A. The effect of interleukin-6 and the interleukin-6 receptor on glucose transport in mouse skeletal muscle. Exp. Physiol. 94, 899–905 (2009).
Tweedell, A. et al. Metabolic response to endotoxin in vivo in the conscious mouse: role of interleukin-6. Metabolism 60, 92–98 (2011).
Pilon, G., Dallaire, P. & Marette, A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J. Biol. Chem. 279, 20767–20774 (2004).
Centeno-Baez, C., Dallaire, P. & Marette, A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am. J. Physiol. Endocrinol. Metab. 301, E922–E930 (2011).
Charbonneau, A. & Marette, A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes 59, 861–871 (2010).
Xu, E. et al. Targeted disruption of carcinoembryonic antigen-related cell adhesion molecule 1 promotes diet-induced hepatic steatosis and insulin resistance. Endocrinology 150, 3503–3512 (2009).
Mari, A. Estimation of the rate of appearance in the non-steady state with a two-compartment model. Am. J. Physiol. 263, E400–E415 (1992).
Mulvihill, E.E. et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 60, 1446–1457 (2011).
Xu, E. et al. Hepatocyte-specific Ptpn6 deletion protects from obesity-linked hepatic insulin resistance. Diabetes 61, 1949–1958 (2012).
Chung, J. et al. HSP72 protects against obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 105, 1739–1744 (2008).
Jenkins, Y. et al. AMPK activation through mitochondrial regulation results in increased substrate oxidation and improved metabolic parameters in models of diabetes. PLoS ONE 8, e81870 (2013).
Moh, A. et al. STAT3 sensitizes insulin signaling by negatively regulating glycogen synthase kinase-3 β. Diabetes 57, 1227–1235 (2008).
Río, A. et al. Reduced liver injury in the interleukin-6 knockout mice by chronic carbon tetrachloride administration. Eur. J. Clin. Invest. 38, 306–316 (2008).
Schmittgen, T.D. & Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 (2008).
Acknowledgements
We thank C. Dion for surgical preparation of the mice and S. Pelletier for assistance with the palmitate macrophage studies. We also thank C. Serhan (Brigham and Women's Hospital, Harvard University) and N. Flamand (Heart and Lung Institute, Laval University) for providing PD1 and (8S,15S)-diHETE, respectively, and M. Schwab and K. Bellmann for their help with the PCR analyses. T37i fibroblasts were a gift from M. Lombès, INSERM U478. This work was supported by grants to A.M. from the Canadian Diabetes Association and from the Canadian Institutes of Health Research (CIHR). A.M. is partially funded by a CIHR/Pfizer Chair in the pathogenesis of insulin resistance and cardiovascular diseases. P.J.W. is the recipient of a PhD studentship award from the Fonds de la Recherche en Santé du Quebec.
Author information
Authors and Affiliations
Contributions
P.J.W. and A.M. conceived of the study and wrote the manuscript. P.J.W., P.S.-P., A.C., P.L.M. and E.S.-A. performed mouse experiments. P.J.W., P.L.M., B.M. and E.S.-A. performed cell culture experiments. P.J.W., P.L.M., E.S.-A. and B.M. conducted ELISA and multiplex analyses. P.J.W. and P.L.M. carried out western blotting and PCR. All authors analyzed and discussed data and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.M. and P.J.W. have filed a patent application (PCT/CA2014/000047) with the Canadian Patent Office describing a method and use for the stimulation of muscular IL-6 secretion.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 3352 kb)
Rights and permissions
About this article
Cite this article
White, P., St-Pierre, P., Charbonneau, A. et al. Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat Med 20, 664–669 (2014). https://doi.org/10.1038/nm.3549
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3549
This article is cited by
-
Protectin DX as a therapeutic strategy against frailty in mice
GeroScience (2023)
-
Maresin 1 regulates insulin signaling in human adipocytes as well as in adipose tissue and muscle of lean and obese mice
Journal of Physiology and Biochemistry (2021)
-
Protectin DX ameliorates inflammation in sepsis-induced acute lung injury through mediating PPARγ/NF-κB pathway
Immunologic Research (2020)
-
Individual differences in EPA and DHA content of Atlantic salmon are associated with gene expression of key metabolic processes
Scientific Reports (2019)
-
Maresin 1 mitigates liver steatosis in ob/ob and diet-induced obese mice
International Journal of Obesity (2018)