Abstract
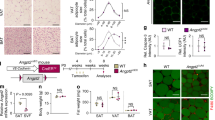
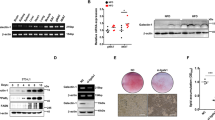
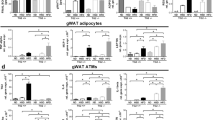
Fatty acids are integral mediators of energy storage, membrane formation and cell signaling. The pathways that orchestrate uptake of fatty acids remain incompletely understood. Expression of the integrin ligand Mfge8 is increased in human obesity and in mice on a high-fat diet, but its role in obesity is unknown. We show here that Mfge8 promotes the absorption of dietary triglycerides and the cellular uptake of fatty acid and that Mfge8-deficient (Mfge8−/−) mice are protected from diet-induced obesity, steatohepatitis and insulin resistance. Mechanistically, we found that Mfge8 coordinates fatty acid uptake through αvβ3 integrin– and αvβ5 integrin–dependent phosphorylation of Akt by phosphatidylinositide-3 kinase and mTOR complex 2, leading to translocation of Cd36 and Fatp1 from cytoplasmic vesicles to the cell surface. Collectively, our results imply a role for Mfge8 in regulating the absorption and storage of dietary fats, as well as in the development of obesity and its complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Berk, P.D. et al. Selective up-regulation of fatty acid uptake by adipocytes characterizes both genetic and diet-induced obesity in rodents. J. Biol. Chem. 274, 28626–28631 (1999).
Berk, P.D. et al. Uptake of long chain free fatty acids is selectively up-regulated in adipocytes of Zucker rats with genetic obesity and non-insulin-dependent diabetes mellitus. J. Biol. Chem. 272, 8830–8835 (1997).
Stump, D.D., Fan, X. & Berk, P.D. Oleic acid uptake and binding by rat adipocytes define dual pathways for cellular fatty acid uptake. J. Lipid Res. 42, 509–520 (2001).
Anderson, C.M. & Stahl, A. SLC27 fatty acid transport proteins. Mol. Aspects Med. 34, 516–528 (2013).
Stahl, A., Evans, J.G., Pattel, S., Hirsch, D. & Lodish, H.F. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev. Cell 2, 477–488 (2002).
Luiken, J.J. et al. Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am. J. Physiol. Endocrinol. Metab. 282, E491–E495 (2002).
Luiken, J.J. et al. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 52, 1627–1634 (2003).
Atabai, K. et al. Mfge8 is critical for mammary gland remodeling during involution. Mol. Biol. Cell 16, 5528–5537 (2005).
Hanayama, R. et al. Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 (2002).
Greenberg, M.E. et al. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 203, 2613–2625 (2006).
Nandrot, E.F. et al. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA 104, 12005–12010 (2007).
Ryeom, S.W., Sparrow, J.R. & Silverstein, R.L. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J. Cell Sci. 109, 387–395 (1996).
Tandon, N.N., Kralisz, U. & Jamieson, G.A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J. Biol. Chem. 264, 7576–7583 (1989).
Atabai, K. et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J. Clin. Invest. 119, 3713–3722 (2009).
Kudo, M. et al. Mfge8 suppresses airway hyperresponsiveness in asthma by regulating smooth muscle contraction. Proc. Natl. Acad. Sci. USA 110, 660–665 (2013).
Silvestre, J.S. et al. Lactadherin promotes VEGF-dependent neovascularization. Nat. Med. 11, 499–506 (2005).
Aziz, M.M. et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent αvβ3 integrin signaling. J. Immunol. 182, 7222–7232 (2009).
Twito, T., Madeleine, D., Perl-Treves, R., Hillel, J. & Lavi, U. Comparative genome analysis with the human genome reveals chicken genes associated with fatness and body weight. Anim. Genet. 42, 642–649 (2011).
Rankinen, T. et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 14, 529–644 (2006).
Henegar, C. et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 9, R14 (2008).
Aoki, N. et al. Identification and characterization of microvesicles secreted by 3T3–L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology 148, 3850–3862 (2007).
Yu, F. et al. Proteomic analysis of aorta and protective effects of grape seed procyanidin b2 in db/db mice reveal a critical role of milk fat globule epidermal growth factor-8 in diabetic arterial damage. PLoS ONE 7, e52541 (2012).
Cheng, M. et al. Correlation between serum lactadherin and pulse wave velocity and cardiovascular risk factors in elderly patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 95, 125–131 (2012).
Liao, J., Sportsman, R., Harris, J. & Stahl, A. Real-time quantification of fatty acid uptake using a novel fluorescence assay. J. Lipid Res. 46, 597–602 (2005).
Pohl, J., Ring, A., Korkmaz, U., Ehehalt, R. & Stremmel, W. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol. Biol. Cell 16, 24–31 (2005).
Samovski, D., Su, X., Xu, Y., Abumrad, N.A. & Stahl, P.D. Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase. J. Lipid Res. 53, 709–717 (2012).
Bonen, A., Luiken, J.J., Arumugam, Y., Glatz, J.F. & Tandon, N.N. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J. Biol. Chem. 275, 14501–14508 (2000).
Jain, S.S. et al. Additive effects of insulin and muscle contraction on fatty acid transport and fatty acid transporters, FAT/CD36, FABPpm, FATP1, 4 and 6. FEBS Lett. 583, 2294–2300 (2009).
Glatz, J.F., Luiken, J.J. & Bonen, A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 90, 367–417 (2010).
Chabowski, A. et al. Insulin stimulates fatty acid transport by regulating expression of FAT/CD36 but not FABPpm. Am. J. Physiol. Endocrinol. Metab. 287, E781–E789 (2004).
Newburg, D.S. et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 351, 1160–1164 (1998).
Coburn, C.T. et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275, 32523–32529 (2000).
Wu, Q. et al. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol. Cell. Biol. 26, 3455–3467 (2006).
Tanaka, T. et al. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 42, 751–759 (2001).
Bamba, V. & Rader, D.J. Obesity and atherogenic dyslipidemia. Gastroenterology 132, 2181–2190 (2007).
Drover, V.A. et al. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J. Biol. Chem. 283, 13108–13115 (2008).
Drover, V.A. et al. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Invest. 115, 1290–1297 (2005).
Nassir, F., Wilson, B., Han, X., Gross, R.W. & Abumrad, N.A. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 282, 19493–19501 (2007).
Moodley, Y. et al. Macrophage recognition and phagocytosis of apoptotic fibroblasts is critically dependent on fibroblast-derived thrombospondin 1 and CD36. Am. J. Pathol. 162, 771–779 (2003).
Liu, J., DeYoung, S.M., Zhang, M., Cheng, A. & Saltiel, A.R. Changes in integrin expression during adipocyte differentiation. Cell Metab. 2, 165–177 (2005).
Mizejewski, G.J. Role of integrins in cancer: survey of expression patterns. Proc. Soc. Exp. Biol. Med. 222, 124–138 (1999).
Zhao, Y. et al. Tumor αvβ3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 67, 5821–5830 (2007).
Liu, Y., Zuckier, L.S. & Ghesani, N.V. Dominant uptake of fatty acid over glucose by prostate cells: a potential new diagnostic and therapeutic approach. Anticancer Res. 30, 369–374 (2010).
Zheng, D.Q., Woodard, A.S., Fornaro, M., Tallini, G. & Languino, L.R. Prostatic carcinoma cell migration via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 59, 1655–1664 (1999).
Asano, K. et al. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J. Exp. Med. 200, 459–467 (2004).
Poehlman, E.T., Scheffers, J., Gottlieb, S.S., Fisher, M.L. & Vaitekevicius, P. Increased resting metabolic rate in patients with congestive heart failure. Ann. Intern. Med. 121, 860–862 (1994).
Bonetto, A. et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS ONE 6, e22538 (2011).
Rolland, Y., Abellan van Kan, G., Gillette-Guyonnet, S. & Vellas, B. Cachexia versus sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 14, 15–21 (2011).
Strissel, K.J. et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56, 2910–2918 (2007).
Huang, X., Griffiths, M., Wu, J., Farese, R.V. Jr. & Sheppard, D. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol. Cell. Biol. 20, 755–759 (2000).
Su, G. et al. Absence of integrin αvβ3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am. J. Respir. Crit. Care Med. 185, 58–66 (2012).
Takahashi, K. et al. A murine very late activation antigen-like extracellular matrix receptor involved in CD2- and lymphocyte function-associated antigen-1–independent killer-target cell interaction. J. Immunol. 145, 4371–4379 (1990).
Ashkar, S. et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287, 860–864 (2000).
Su, G. et al. Integrin αvβ5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am. J. Respir. Cell Mol. Biol. 36, 377–386 (2007).
Zovein, A.C. et al. β1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev. Cell 18, 39–51 (2010).
Helming, L., Winter, J. & Gordon, S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J. Cell Sci. 122, 453–459 (2009).
Thomas, G.J., Hart, I.R., Speight, P.M. & Marshall, J.F. Binding of TGF-β1 latency-associated peptide (LAP) to αvβ6 integrin modulates behaviour of squamous carcinoma cells. Br. J. Cancer 87, 859–867 (2002).
Tseng, Y.H. et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 (2008).
Huang, P. et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475, 386–389 (2011).
Anwar, K., Iqbal, J. & Hussain, M.M. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J. Lipid Res. 48, 2028–2038 (2007).
Smyth, J.W. et al. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ. Res. 110, 978–989 (2012).
Rabot, S. et al. Germ-free C57BL/6J mice are resistant to high-fat-diet–induced insulin resistance and have altered cholesterol metabolism. FASEB J. 24, 4948–4959 (2010).
Uchida, A. et al. Reduced triglyceride secretion in response to an acute dietary fat challenge in obese compared to lean mice. Front. Physiol. 3, 26 (2012).
Kim, K.Y. et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Invest. 121, 3701–3712 (2011).
Sutton, G.M. et al. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology 147, 2183–2196 (2006).
Ueyama, A., Sato, T., Yoshida, H., Magata, K. & Koga, N. Nonradioisotope assay of glucose uptake activity in rat skeletal muscle using enzymatic measurement of 2-deoxyglucose 6-phosphate in vitro and in vivo. Biol. Signals Recept. 9, 267–274 (2000).
Acknowledgements
This research was supported by US National Institutes of Health grant P30 DK 063-7202 and the University of California, San Francisco (UCSF) Diabetes and Endocrinology Research Center and UCSF Cardiovascular Research Institute startup funds (K.A.). We would like to thank K. Nguyen for help with flow cytometry studies, A. Atakilit and D. Sheppard (UCSF Lung Biology Center) for providing integrin-blocking antibodies, R. Silverstein for providing Cd36−/− mice (Cleveland Clinic Research Institute) and K. Ashrafi for thoughtful review of the manuscript.
Author information
Authors and Affiliations
Contributions
A.K.-S. and W.M. designed and performed in vivo and in vitro experiments and aided in writing the manuscript. S.S. carried out in vivo experiments with obese mice, isolated recombinant protein constructs and performed flow cytometry. Y.Y.C. carried out in vivo insulin sensitivity studies. K.T. aided with isolation of primary and pre-adipocytes and measured fecal energy content. Y.Q. aided with metabolic cage studies. S.M.T., A.S. and A.C. aided in the design of experiments and interpretation of results. K.A. designed the study, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 26356 kb)
Rights and permissions
About this article
Cite this article
Khalifeh-Soltani, A., McKleroy, W., Sakuma, S. et al. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat Med 20, 175–183 (2014). https://doi.org/10.1038/nm.3450
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3450
This article is cited by
-
iPSCs derived from insulin resistant offspring of type 2 diabetic patients show increased oxidative stress and lactate secretion
Stem Cell Research & Therapy (2022)
-
High-protein diet prevents fat mass increase after dieting by counteracting Lactobacillus-enhanced lipid absorption
Nature Metabolism (2022)
-
Hepatocyte ATF3 protects against atherosclerosis by regulating HDL and bile acid metabolism
Nature Metabolism (2021)
-
Angiopoietin-2–integrin α5β1 signaling enhances vascular fatty acid transport and prevents ectopic lipid-induced insulin resistance
Nature Communications (2020)
-
A rare missense variant in the milk fat globule-EGF factor 8 (MFGE8) increases T2DM susceptibility and cardiovascular disease risk with population-specific effects
Acta Diabetologica (2020)