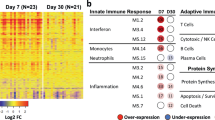
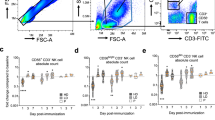
Abstract
Measles virus (MV) still incites one of the most contagious infections of humankind. Despite the development and use of an excellent live attenuated virus vaccine, over one million infants and children continue to die each year from measles1–3. The main cause of morbidity and mortality is virus–induced immunosuppression of lymphocyte function, which allows secondary infections. Here we report an in vivo model for the study of MV–induced immunosuppression. Human peripheral blood leukocytes (PBLs) grafted onto mice with severe combined immunodeficiency disease (SCID mice) to create hu–PBLS–SCID mice produce human IgG that is suppressed by MV infection, immunosuppression is dependent on the involvement of live virus and is dramatically more severe for PBLs obtained from newborns than PBLs from adults. Suppression of IgG synthesis by PBLs from newborns occurs as early as ten days after administration of MV to hu–PBLS–SCID mice compared with 44 days required for PBLs from adults. Further, MV infection of SCID mice reconstituted with PBLs from newborns reduces IgG production 26 ± 5–fold (mean ± 1 s.e.m.) as compared with only a 6 ± 0.5–fold reduction in adults. MV RNA could be detected in live human PBLs recovered from SCID mice as long as 110 days after MV infection began. The profound immunosuppression we observe in PBLs from infants probably contributes to the morbidity and mortality observed in infants vaccinated with measles virus. Further, this model should be useful for accessing the potential immunosuppressive abilities of newly isolated field (wild–type) virus isolates and newly designed vaccines containing attenuated MV or subunit vaccines, as well as in dissecting the role played by maternal antibodies to MV on the ability of the virus to enhance or abort the virus–induced immunosuppression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Griffin, D.E. .& Bellini, W.J. Measles virus. in Virology (eds. Fields, B.N., Knipe, D.M. & Howley, P.M.) 1267–1312 (Lippincott-Raven, Philadelphia, 1996).
Markowitz, L. Katz, S. Measles vaccine. in Vaccines (eds. Plotkin, S. & Mortimer, S.) 229–276 (Saunders, Philadelphia, 1994).
Black, F.L. Measles active and passive immunity in a worldwide perspective. Prog. Med. Virol. 36, 1–33 (1989).
Hendrickson, E.A. The SCID mouse: Relevance as an animal model system for studying human disease. Am. J. Pathol. 143, 1511–1522 (1993).
Bosma, G.C., Custer, R.P. & Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 301, 527–530 (1983).
Mosier, D., Gulizia, R., Baird, S. & Wilson, D. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 335, 256–9 (1988).
Duchosal, M.A., Eming, S.A., McConahey, P.J. & Dixon, F.J. The hu-PBLS-SCID mouse model: Long-term human serologic evolution associated with the xenogeneic transfer of human peripheral blood leukocytes into SCID mice. Cell. Immunol. 139, 468–477 (1992).
Mosier, D.E. et al. Human immunodeficiency virus infection of human-PBLS-SC1D mice. Science 251, 791–794 (1991).
Mosier, D., Gulizia, R., Maclsaac, P., Torbett, B. & Levy, J. Rapid loss of CD4+ T cells in human-PBLS-SCID mice by noncytopathic HIV isolates. Science 260, 689–692 (1993).
Mosier, D.E., Gulizia, R.J., Maclsaac, P.O., Corey, L. & Greenberg, P.O. Resistance to human immunodeficiency virus 1 infection of SCID mice reconstituted with peripheral blood leukocytes from donors vaccinated with vaccinia gp160 and recombinant gp160. Proc. Natl Acad. Sd. USA 90, 2443–7 (1993).
Tyor, W., Power, C., Gendelman, H. & Markham, R. A model of human immunodeficiency virus encephalitis in scid mice. Proc. Natl. Acad. Sci. USA 90, 8658–8662 (1993).
Cannon, M.P., Pisa, P., Fox, R. & Cooper, N. Epstein-Barr virus induces aggressive lymphoproliferative disorders of human B cell origin in SCID/hu chimeric mice. J. Clin din. Invest. 85, 1333–1337 (1990).
Mocarski, E., Bonyhadi, M., Salimi, S., McCune, J.M. & Kaneshima, H. Human cytomegalovirus in a SCID-hu mouse: Thymic epithelial cells are prominent targets of viral replication. Proc. Natl. Acad. Sci. USA 90, 104–108 (1993).
Naniche, D. et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67, 6025–6032 (1993).
Dorig, R., Marcel, A., Chopra, A. & Richardson, C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75, 295–305 (1993).
Manchester, M., Liszewski, M.K., Atkinson, J.P. & Oldstone, M.B.A. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc. Natl. Acad. Sci. USA 91, 2161–2165 (1994).
Slifka, M.K., Matloubian, M. & Ahmed, R. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69, 1895–902 (1995).
Ahmed, R. & Gray, D. Immunologic memory and protective immunity: Understanding their relation. Science 272, 54–60 (1996).
Ueki, Y. et al Clonal analysis of a human antibody response. Quantification of precursors and antibody producing cells and generation and characterization of monoclonal IgM, IgG, and IgA to rabies virus. J. Exp. Med. 171, 19–34 (1990).
McChesney, M.B. & Oldstone, M.B.A. Virus-induced immunosuppression: Infections with measles virus and human immunodeficiency virus. Adv. Immunol. 45, 335–380 (1989).
Yanagi, Y., Cubitt, B. & Oldstone, M.B.A. Measles virus inhibits mitogen-in-duced T cell proliferation but does not directly perturb the T cell activation process inside the cell. Virology 187, 280–289 (1992).
von Pirquet, C.E. Das Verhalten der kutanen Tuberkulin-reaktion wahrend der Masern. Deutsch Med. Wochenschr. 34, 1297–1300 (1908).
McChesney, M.B., Fujinami, R.S., Lampert, P.W. & Oldstone, M.B.A. Viruses disrupt functions of human T lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J. Exp. Med. 163, 1331–1336 (1986).
McChesney, M.B., Kehrl, J.H., Valsamakis, A., Fauci, A.S. & Oldstone, M.B.A. Measles virus infection of B lymphocytes permits cellular activation but blocks progression through the cell cycle. J. Virol. 61, 3441–3447 (1987).
McChesney, M.B., Altman, A. & Oldstone, M.B.A. Suppression of T lymphocyte function by measles virus is due to cell cycle arrest in G1. J. Immunol. 140, 1269–1273 (1988).
Casali, P., Rice, G.P.A. & Oldstone, M.B.A. Viruses disrupt functions of human lymphocytes: Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J. Exp. Med. 159, 1322–1337 (1984).
Fenner, F., McAuslan, B.R., Minis, C.A., Sambrook, J. & White, D. The Biology of Animal Viruses (Academic Press, New York, 1974).
McChesney, M.B., Fujinami, R.S., Lerche, N.W., Marx, P.A. & Oldstone, M.B.A. Virus induced immunosuppression: Infection of peripheral blood mononuclear cells and suppression of immunoglobulin synthesis during natural measles virus infection of rhesus monkeys. J. Infect. Dis. 159, 757–760 (1989).
Rebai, N. & Malissen, B. Structural and genetic analyses of HLA class 1 molecules using monoclonal xenoantibodies. Tissue Antigens 22, 107–117 (1983).
Meizlik, E.H. & Carpenter, A.M. Beneficial effect of measles on nephrosis: Report of 3 cases. Am. J. Dis. Child. 76, 83–90 (1948).
Gellen, B. & Katz, S. Putting a stop to a serial killer: Measles. J. Infect. Dis. 170, S1–S3 (1994).
Markowitz, L.E. Bernier, H. Immunization of younginfantswith Edmonston-Zagreb measles vaccine. Pediatr. Infect. Dis. J. 6, 809–812 (1987).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular Cloning: A Laboratory Manual edn. 2 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tishon, A., Manchester, M., Scheiflinger, F. et al. A model of measles virus–induced immunosuppression: Enhanced susceptibility of neonatal human PBLs. Nat Med 2, 1250–1254 (1996). https://doi.org/10.1038/nm1196-1250
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm1196-1250
This article is cited by
-
Effect of methanolic extract of Mucuna pruriens seed on the immune response of mice
Comparative Clinical Pathology (2012)
-
Negative signaling in health and disease
Immunologic Research (1999)