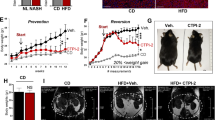
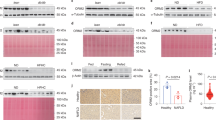
Abstract
Increased hepatic lipid content is an early correlate of insulin resistance and can be caused by nutrient-induced activation of mammalian target of rapamycin (mTor). This activation of mTor increases basal Akt activity, leading to a self-perpetuating lipogenic cycle. We have previously shown that the developmental Notch pathway has metabolic functions in adult mouse liver. Acute or chronic inhibition of Notch dampens hepatic glucose production and increases Akt activity and may therefore be predicted to increase hepatic lipid content. Here we now show that constitutive liver-specific ablation of Notch signaling, or its acute inhibition with a decoy Notch1 receptor, prevents hepatosteatosis by blocking mTor complex 1 (mTorc1) activity. Conversely, Notch gain of function causes fatty liver through constitutive activation of mTorc1, an effect that is reversible by treatment with rapamycin. We demonstrate that Notch signaling increases mTorc1 complex stability, augmenting mTorc1 function and sterol regulatory element binding transcription factor 1c (Srebp1c)-mediated lipogenesis. These data identify Notch as a therapeutically actionable branch point of metabolic signaling at which Akt activation in the liver can be uncoupled from hepatosteatosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wang, Y.C., McPherson, K., Marsh, T., Gortmaker, S.L. & Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378, 815–825 (2011).
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004).
Li, S., Brown, M.S. & Goldstein, J.L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA 107, 3441–3446 (2010).
Sabatini, D.M. mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer 6, 729–734 (2006).
Peterson, T.R. et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420 (2011).
Matsumoto, M., Pocai, A., Rossetti, L., Depinho, R.A. & Accili, D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 6, 208–216 (2007).
Matsumoto, M., Han, S., Kitamura, T. & Accili, D. Dual role of transcription factor Foxo1 in controlling hepatic insulin sensitivity and lipid metabolism. J. Clin. Invest. 116, 2464–2472 (2006).
Savage, D.B. & Semple, R.K. Recent insights into fatty liver, metabolic dyslipidaemia and their links to insulin resistance. Curr. Opin. Lipidol. 21, 329–336 (2010).
Bolós, V., Grego-Bessa, J. & de la Pompa, J.L. Notch signaling in development and cancer. Endocr. Rev. 28, 339–363 (2007).
Fortini, M.E. Notch signaling: the core pathway and its posttranslational regulation. Dev. Cell 16, 633–647 (2009).
Dufraine, J., Funahashi, Y. & Kitajewski, J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene 27, 5132–5137 (2008).
Swiatek, P.J., Lindsell, C.E., del Amo, F.F., Weinmaster, G. & Gridley, T. Notch1 is essential for postimplantation development in mice. Genes Dev. 8, 707–719 (1994).
Oka, C. et al. Disruption of the mouse RBP-Jκ gene results in early embryonic death. Development 121, 3291–3301 (1995).
Shen, J. et al. Skeletal and CNS defects in Presenilin-1–deficient mice. Cell 89, 629–639 (1997).
Rizzo, P. et al. Rational targeting of Notch signaling in cancer. Oncogene 27, 5124–5131 (2008).
Weinmaster, G. & Kopan, R. A garden of Notch-ly delights. Development 133, 3277–3282 (2006).
Pajvani, U.B. et al. Inhibition of Notch signaling ameliorates insulin resistance in a Foxo1-dependent manner. Nat. Med. 17, 961–967 (2011).
Chan, S.M., Weng, A.P., Tibshirani, R., Aster, J.C. & Utz, P.J. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood 110, 278–286 (2007).
Efferson, C.L. et al. Downregulation of Notch pathway by a γ-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res. 70, 2476–2484 (2010).
Postic, C. & Magnuson, M.A. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26, 149–150 (2000).
Kitamura, T. et al. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J. Clin. Invest. 117, 2477–2485 (2007).
Haeusler, R.A., Pratt-Hyatt, M., Welch, C.L., Klaassen, C.D. & Accili, D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 15, 65–74 (2012).
Tao, R. et al. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J. Biol. Chem. 286, 14681–14690 (2011).
Postic, C. & Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 118, 829–838 (2008).
Kim, J.B., Wright, H.M., Wright, M. & Spiegelman, B.M. ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proc. Natl. Acad. Sci. USA 95, 4333–4337 (1998).
Zhang, Y.L. et al. Aberrant hepatic expression of PPARγ2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J. Biol. Chem. 281, 37603–37615 (2006).
Gingras, A.C. et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 13, 1422–1437 (1999).
Chiang, G.G. & Abraham, R.T. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 280, 25485–25490 (2005).
Weng, Q.P. et al. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J. Biol. Chem. 273, 16621–16629 (1998).
Funahashi, Y. et al. A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res. 68, 4727–4735 (2008).
Funahashi, Y. et al. Notch modulates VEGF action in endothelial cells by inducing matrix metalloprotease activity. Vasc. Cell 3, 2 (2011).
Peterson, T.R. et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137, 873–886 (2009).
Blättler, S.M. et al. Yin Yang 1 deficiency in skeletal muscle protects against rapamycin-induced diabetic-like symptoms through activation of insulin/IGF signaling. Cell Metab. 15, 505–517 (2012).
Kim, D.H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002).
Foster, K.G. et al. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J. Biol. Chem. 285, 80–94 (2010).
Kaizuka, T. et al. Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J. Biol. Chem. 285, 20109–20116 (2010).
Liu, H. et al. Wnt signaling regulates hepatic metabolism. Sci. Signal. 4, ra6 (2011).
Haeusler, R.A., Kaestner, K.H. & Accili, D. FoxOs function synergistically to promote glucose production. J. Biol. Chem. 285, 35245–35248 (2010).
Sun, Z. et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat. Med. 18, 934–942 (2012).
Hagiwara, A. et al. Hepatic mTorc2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 15, 725–738 (2012).
Fukuda, D. et al. Notch ligand Delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc. Natl. Acad. Sci. USA 109, E1868–E1877 (2012).
Kim-Muller, J.Y. & Accili, D. Cell biology. Selective insulin sensitizers. Science 331, 1529–1531 (2011).
Howell, J.J. & Manning, B.D. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 22, 94–102 (2011).
Yecies, J.L. et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 14, 21–32 (2011).
Gwinn, D.M. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008).
Noguera-Troise, I. et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444, 1032–1037 (2006).
Wu, Y. et al. Therapeutic antibody targeting of individual Notch receptors. Nature 464, 1052–1057 (2010).
Houde, V.P. et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 59, 1338–1348 (2010).
van Es, J.H. et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005).
Fujikura, J. et al. Notch/Rbp-j signaling prevents premature endocrine and ductal cell differentiation in the pancreas. Cell Metab. 3, 59–65 (2006).
Paik, J.H. et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309–323 (2007).
Folch, J., Lees, M. & Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Millar, J.S., Cromley, D.A., McCoy, M.G., Rader, D.J. & Billheimer, J.T. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J. Lipid Res. 46, 2023–2028 (2005).
Li, G., Hernandez-Ono, A., Crooke, R.M., Graham, M.J. & Ginsberg, H.N. Effects of antisense-mediated inhibition of 11β-hydroxysteroid dehydrogenase type 1 on hepatic lipid metabolism. J. Lipid Res. 52, 971–981 (2011).
Nakae, J. et al. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell 4, 119–129 (2003).
Kim, J.B. et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Invest. 101, 1–9 (1998).
Qiang, L. et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 150, 620–632 (2012).
Acknowledgements
This work was supported by US National Institutes of Health grants DK093604 (U.B.P.), DK57539 (D.A.), HL062454 (J.K.) and DK63608 (Columbia Diabetes Research Center). We thank D. Conlon, C. Eng, I. Goldberg, R. Haeusler and I. Tabas, as well as members of the Accili, Kitajewski and Ginsberg laboratories, for insightful discussion of the data. We acknowledge excellent technical support from A. Flete, T. Kolar and J. Lee, as well as plasmids from D. Sabatini (Whitehead Institute) and B. Spiegelman (Dana-Farber Cancer Institute).
Author information
Authors and Affiliations
Contributions
U.B.P. designed and performed experiments, analyzed data and wrote the manuscript. L.Q. and T.K. designed and performed experiments and analyzed data. J.K., H.N.G. and D.A. designed the studies, analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 925 kb)
Rights and permissions
About this article
Cite this article
Pajvani, U., Qiang, L., Kangsamaksin, T. et al. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med 19, 1054–1060 (2013). https://doi.org/10.1038/nm.3259
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3259
This article is cited by
-
Targeted inhibition of RBPJ transcription complex alleviates the exhaustion of CD8+ T cells in hepatocellular carcinoma
Communications Biology (2023)
-
ZFYVE28 mediates insulin resistance by promoting phosphorylated insulin receptor degradation via increasing late endosomes production
Nature Communications (2023)
-
LKB1 on POMC neurons affect the formation of diet-induced obesity by regulating the expression of HDAC1
Genes & Genomics (2022)
-
Structural and functional characterization of disease-associated NOTCH4: a potential modulator of PI3K/AKT-mediated insulin signaling pathway
Applied Nanoscience (2022)
-
Maladaptive regeneration — the reawakening of developmental pathways in NASH and fibrosis
Nature Reviews Gastroenterology & Hepatology (2021)