Abstract
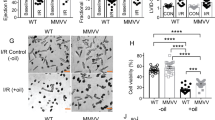
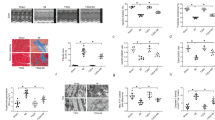
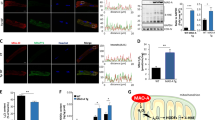
Oxidative damage from elevated production of reactive oxygen species (ROS) contributes to ischemia-reperfusion injury in myocardial infarction and stroke. The mechanism by which the increase in ROS occurs is not known, and it is unclear how this increase can be prevented. A wide variety of nitric oxide donors and S-nitrosating agents protect the ischemic myocardium from infarction, but the responsible mechanisms are unclear1,2,3,4,5,6. Here we used a mitochondria-selective S-nitrosating agent, MitoSNO, to determine how mitochondrial S-nitrosation at the reperfusion phase of myocardial infarction is cardioprotective in vivo in mice. We found that protection is due to the S-nitrosation of mitochondrial complex I, which is the entry point for electrons from NADH into the respiratory chain. Reversible S-nitrosation of complex I slows the reactivation of mitochondria during the crucial first minutes of the reperfusion of ischemic tissue, thereby decreasing ROS production, oxidative damage and tissue necrosis. Inhibition of complex I is afforded by the selective S-nitrosation of Cys39 on the ND3 subunit, which becomes susceptible to modification only after ischemia. Our results identify rapid complex I reactivation as a central pathological feature of ischemia-reperfusion injury and show that preventing this reactivation by modification of a cysteine switch is a robust cardioprotective mechanism and hence a rational therapeutic strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Duranski, M.R. et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Invest. 115, 1232–1240 (2005).
Shiva, S. et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 204, 2089–2102 (2007).
Lundberg, J.O., Weitzberg, E. & Gladwin, M.T. The nitrate-nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7, 156–167 (2008).
Methner, C., Schmidt, K., Cohen, M.V., Downey, J.M. & Krieg, T. Both A2a and A2b adenosine receptors at reperfusion are necessary to reduce infarct size in mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 299, H1262–H1264 (2010).
Cohen, M.V., Yang, X.M., Liu, Y.P., Solenkova, N.V. & Downey, J.M. Cardioprotective PKG-independent NO signaling at reperfusion. Am. J. Physiol. Heart Circ. Physiol. 299, H2028–H2036 (2010).
Costa, A.D. et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ. Res. 97, 329–336 (2005).
Bolli, R. et al. Myocardial protection at a crossroads. Circ. Res. 95, 125–134 (2004).
Yellon, D.M. & Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 357, 1121–1135 (2007).
Turer, A.T. & Hill, J.A. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am. J. Cardiol. 106, 360–368 (2010).
Sun, J., Morgan, M., Shen, R.F., Steenbergen, C. & Murphy, E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ. Res. 101, 1155–1163 (2007).
Prime, T.A. et al. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 106, 10764–10769 (2009).
Chouchani, E.T. et al. Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol difference in gel electrophoresis (SNO-DIGE): implications for the regulation of mitochondrial function by reversible S-nitrosation. Biochem. J. 430, 49–59 (2010).
Murray, C.I., Kane, L.A., Uhrigshardt, H., Wang, S.B. & Van Eyk, J.E. Site-mapping of in vitro S-nitrosation in cardiac mitochondria: implications for cardioprotection. Mol. Cell Prot. 10, M110.004721 (2011).
Porteous, C.M. et al. Rapid uptake of lipophilic triphenylphosphonium cations by mitochondria in vivo following intravenous injection: implications for mitochondria-specific therapies and probes. Biochim. Biophys. Acta 1800, 1009–1017 (2010).
Wang, X., Kettenhofen, N.J., Shiva, S., Hogg, N. & Gladwin, M.T. Copper dependence of the biotin switch assay: modified assay for measuring cellular and blood nitrosated proteins. Free Radic. Biol. Med. 44, 1362–1372 (2008).
Kettenhofen, N.J., Wang, X., Gladwin, M.T. & Hogg, N. In-gel detection of S-nitrosated proteins using fluorescence methods. Methods Enzymol. 441, 53–71 (2008).
Chouchani, E.T., James, A.M., Fearnley, I.M., Lilley, K.S. & Murphy, M.P. Proteomic approaches to the characterization of protein thiol modification. Curr. Opin. Chem. Biol. 15, 120–128 (2011).
Methner, C., Lukowski, R., Hofman, F., Murphy, M.P. & Krieg, T. Protection through postconditioning or a mitochondria-targeted S-nitrosothiol is unaffected by cardiomyocyte-selective ablation of protein kinase G. Basic Res. Cardiol. 108, 337 (2013).
Murphy, E. & Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 88, 581–609 (2008).
Cochemé, H.M. et al. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340–350 (2011).
Clementi, E., Brown, G.C., Feelisch, M. & Moncada, S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. USA 95, 7631–7636 (1998).
Burwell, L.S., Nadtochiy, S.M., Tompkins, A.J., Young, S. & Brookes, P.S. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem. J. 394, 627–634 (2006).
Dahm, C.C., Moore, K. & Murphy, M.P. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite. J. Biol. Chem. 281, 10056–10065 (2006).
Galkin, A. & Moncada, S. S-nitrosation of mitochondrial complex I depends on its structural conformation. J. Biol. Chem. 282, 37448–37453 (2007).
Carroll, J. et al. Bovine complex I is a complex of 45 different subunits. J. Biol. Chem. 281, 32724–32727 (2006).
Galkin, A. et al. Identification of the mitochondrial ND3 subunit as a structural component involved in the active/deactive enzyme transition of respiratory complex I. J. Biol. Chem. 283, 20907–20913 (2008).
Ciano, M., Fuszard, M., Heide, H., Botting, C.H. & Galkin, A. Conformation-specific crosslinking of mitochondrial complex I. FEBS Lett. 587, 867–872 (2013).
Gorenkova, N., Robinson, E., Grieve, D.J. & Galkin, A. Conformational change of mitochondrial complex I increases ROS sensitivity during ischemia. Antioxid. Redox Signal. published online, http://dx.doi.org/10.1089/ARS.2012.4698 (29 March 2013).
Held, J.M. et al. Targeted quantitation of site-specific cysteine oxidation in endogenous proteins using a differential alkylation and multiple reaction monitoring mass spectrometry approach. Mol. Cell. Proteomics 9, 1400–1410 (2010).
Requejo, R. et al. Measuring mitochondrial protein thiol redox state. Methods Enzymol. 474, 123–147 (2010).
Bridges, H.R., Birrell, J.A. & Hirst, J. The mitochondrial-encoded subunits of respiratory complex I (NADH:ubiquinone oxidoreductase): identifying residues important in mechanism and disease. Biochem. Soc. Trans. 39, 799–806 (2011).
Sazanov, L.A. & Hinchcliffe, P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 311, 1430–1436 (2006).
Efremov, R.G. & Sazanov, L.A. Structure of the membrane domain of respiratory complex I. Nature 476, 414–420 (2011).
Baradaran, R., Berrisford, J.M., Minhas, G.S. & Sazanov, L.A. Crystal structure of the entire respiratory complex I. Nature 494, 443–448 (2013).
Guo, S. et al. A cell-based phenotypic assay to identify cardioprotective agents. Circ. Res. 110, 948–957 (2012).
Mimaki, M. et al. Understanding mitochondrial complex I assembly in health and disease. Biochim. Biophys. Acta 1817, 851–862 (2012).
Shiva, S. & Gladwin, M.T. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Res. Cardiol. 104, 113–119 (2009).
Webb, A. et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. USA 101, 13683–13688 (2004).
Sun, J. et al. Essential role of nitric oxide in acute ischemic preconditioning: S-nitros(yl)ation versus sGC/cGMP/PKG signaling? Free Radic. Biol. Med. 54, 105–112 (2013).
Nadtochiy, S.M., Redman, E., Rahman, I. & Brookes, P.S. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc. Res. 89, 643–649 (2011).
Schmidt, K. et al. Cardioprotective effects of mineralocorticoid receptor antagonists at reperfusion. Eur. Heart J. 31, 1655–1662 (2010).
Chappell, J.B. & Hansford, R.G. in Subcellular Components: Preparation and Fractionation (ed. Birnie, G.D.) 77 (Butterworths, London, 1972).
Perkins, D.N., Pappin, D.J., Creasy, D.M. & Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 (1999).
Wittig, I., Braun, H.P. & Schagger, H. Blue native PAGE. Nat. Protoc. 1, 418–428 (2006).
Rais, I., Karas, M. & Schagger, H. Two-dimensional electrophoresis for the isolation of integral membrane proteins and mass spectrometric identification. Proteomics 4, 2567–2571 (2004).
Srere, P.A. Citrate synthase. Methods Enzymol. 13, 3–10 (1969).
Birrell, J.A., Yakovlev, G. & Hirst, J. Reactions of the flavin mononucleotide in complex I: a combined mechanism describes NADH oxidation coupled to the reduction of APAD+, ferricyanide, or molecular oxygen. Biochemistry 48, 12005–12013 (2009).
Acknowledgements
This study was supported by the UK Medical Research Council and grants from the UK Biotechnology and Biological Sciences Research Council (BB/I012923 to M.P.M. and R.C.H.), the Gates Cambridge Trust and the Canadian Institutes of Health Research (doctoral scholarship and postdoctoral fellowship to E.T.C.), the British Heart Foundation (PG/12/42/29655 to T.K.), the US National Institutes of Health (R01-HL071158 to P.S.B.) and the International Society for Heart Research (ISHR-ES/SERVIER research fellowship to C.M.). We thank L. Sazanov and J. Hirst for helpful discussions.
Author information
Authors and Affiliations
Contributions
E.T.C. designed research, carried out mass spectrometry, fluorescence and biochemical experiments, analyzed data from in vivo experiments and cowrote the paper. C.M., S.M.N. and V.R.P. carried out and analyzed data from in vivo experiments. J.R. assisted with DIGE experiments. A.L. and S.D. carried out mass spectrometry experiments. A.M.J. and H.M.C. assisted with research design and data interpretation. A.J.R. designed and performed bioinformatic experiments. L.P., I.M.F., R.C.H., K.S.L., R.A.J.S., T.K. and P.S.B. assisted with research design. M.P.M. designed and directed the project and cowrote the paper.
Corresponding author
Ethics declarations
Competing interests
M.P.M. and R.A.J.S. hold an EU patent on the technology described in this publication.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–17, Supplementary Tables 1–6 and Supplementary Methods (PDF 5447 kb)
Rights and permissions
About this article
Cite this article
Chouchani, E., Methner, C., Nadtochiy, S. et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19, 753–759 (2013). https://doi.org/10.1038/nm.3212
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3212
This article is cited by
-
Mitochondrial heterogeneity in diseases
Signal Transduction and Targeted Therapy (2023)
-
Preventing mitochondrial reverse electron transport as a strategy for cardioprotection
Basic Research in Cardiology (2023)
-
Mechanisms of mitochondrial respiratory adaptation
Nature Reviews Molecular Cell Biology (2022)
-
In vivo mitochondrial base editing via adeno-associated viral delivery to mouse post-mitotic tissue
Nature Communications (2022)
-
Plant-specific features of respiratory supercomplex I + III2 from Vigna radiata
Nature Plants (2022)