Abstract
Early data emerging from the first phase 3 trial of a malaria vaccine are raising hopes that a licensed vaccine will soon be available for use in endemic countries, but given the relatively low efficacy of the vaccine, this needs to be seen as a major step forward on the road to a malaria vaccine rather than as arrival at the final destination. The focus for vaccine developers now moves to the next generation of malaria vaccines, but it is not yet clear what characteristics these new vaccines should have or how they can be evaluated. Here we briefly review the epidemiological and immunological requirements for malaria vaccines and the recent history of malaria vaccine development and then put forward a manifesto for future research in this area. We argue that rational design of more effective malaria vaccines will be accelerated by a better understanding of the immune effector mechanisms involved in parasite regulation, control and elimination.
Similar content being viewed by others
Main
During the 20th century, numerous highly effective vaccines with enormous benefits for human and animal health were produced against pathogens characterized by single or infrequent infection and lifelong immunity. Developing vaccines against pathogens that cause chronic infections has been much more difficult. Three of these pathogens, HIV, malaria and tuberculosis, are international health priorities for the 21st century. In each case, progress is slow, sporadic and unpredictable, but much basic knowledge about the pathogens and their hosts has been acquired. For malaria, a long and painstaking program of research has finally yielded one vaccine candidate that, although far from ideal, has the potential to reduce childhood morbidity in Africa and beyond. It seems opportune at this time to reflect on the lessons we have learned from the last three decades of malaria vaccine research and consider alternate approaches to the development of more effective, second-generation vaccines.
Why do we need a malaria vaccine, and what should it do?
Despite recent progress in reducing the burden of malaria in some areas of seasonal or sporadic malaria transmission, malaria remains an intractable problem in much of Africa. Malarial disease disproportionately affects the poor, children under the age of 5 years and pregnant women. The World Health Organization estimates that that there were 216 million clinical episodes and 655,000 deaths from malaria in 2010 (ref. 1), although a recent systematic analysis indicated that the global death toll exceeded 1.2 million in 2010 (refs. 2,3). The malaria burden is increased further by enhanced susceptibility to other infections and lifelong health effects of exposure to malaria before birth3. Moreover, a substantial number of cases occur outside of Africa in densely populated countries of the Indian subcontinent and southeast Asia, emphasizing the need for strategies to control species other than Plasmodium falciparum, such as Plasmodium vivax and, increasingly, Plasmodium knowlesi4, which are endemic in these areas.
Given the enormous genetic and genomic plasticity of malaria parasites, the emergence of antimalarial drug resistance is inevitable. Reports of emerging resistance to the newest family of antimalarial drugs—the artemisinins—are particularly worrying5, and drug discovery is struggling to keep ahead of the spread of resistance6. Moreover, insecticide resistance is again becoming a problem7. This has led to an acceptance that sustainable reductions in malaria morbidity and mortality will require the development and deployment of drugs and vaccines to prevent the transmission of malaria from human to mosquito, thus interrupting the sexual phase of the parasite life cycle in which new (resistant and multiresistant) genotypes emerge8 (Fig. 1). This creates an immediate conundrum for those working on malaria vaccines: should they focus on developing vaccines that reduce morbidity and mortality but have little impact on transmission (thereby saving lives in the short term) or on those designed primarily to interrupt transmission (thereby protecting only at the population level)? Ideally, both aims should be achieved.
infections, with the main immune responses that control the parasite at each stage. Sporozoites, injected into the skin by the biting mosquito, drain to the lymph nodes, where they prime T and B cells, or the liver, where they invade hepatocytes. Antibodies (Ab) trap sporozoites in the skin or prevent their invasion of liver cells. IFN-γ–producing CD4+ and CD8+ T cells inhibit parasite development into merozoites inside the hepatocyte. However, this immune response is frequently insufficient, and merozoites emerging from the liver invade red blood cells, replicate, burst out of the infected erythrocyte and invade new erythrocytes. Merozoite-specific antibodies agglutinate and opsonize the parasite and can inhibit the invasion of red blood cells through receptor blockade. Antibodies to variant surface proteins also opsonize and agglutinate infected red blood cells (RBCs) and prevent their sequestration (cytoadherence) in small blood vessels. IFN-γ–producing lymphocytes activate macrophages and enhance the phagocytosis of opsonized merozoites and iRBCs. Complement-fixing antibodies to gametocyte and gamete antigens lyse parasites inside the mosquito gut or prevent the fertilization and development of the zygote. Sporozoite, liver-stage and gametocyte and gamete antigens are somewhat polymorphic, whereas merozoite antigens and variant surface antigens are highly polymorphic. APC, antigen-presenting cell.
The necessary prioritization of human and financial resources is forcing some difficult decisions to be made. In 2006, the Bill and Melinda Gates Foundation—currently one of the largest funders globally of translational research for malaria control—funded the Malaria Vaccine Technology Roadmap9, a global strategy for accelerating the development and licensing of a highly effective malaria vaccine, which set out two goals for vaccine research: a vaccine that is 50% protective against severe disease and death by 2015, and a vaccine that prevents 80% of clinical malaria episodes by 2025. However, although many major funders, such as the US National Institutes of Health, the Wellcome Trust and the European Commission, continue to fund broad portfolios of vaccine research, the most recent Bill and Melinda Gates Foundation Malaria Strategy document (April 2011) now specifically prioritizes vaccines designed to interrupt malaria transmission10.
The malaria vaccine landscape is extremely heterogeneous, with many disparate approaches being followed; however, for more than a decade, this landscape has been dominated by one particular vaccine candidate. The anticipated licensing of RTS,S/AS01 as the first malaria vaccine, expected in 2015, will substantially affect malaria vaccine research. Assuming that RTS,S will be used in a substantial number of P. falciparum–endemic countries, any new vaccine will need to show markedly greater protective efficacy than the 30–50% reported for RTS,S11,12. In the absence of reliable correlates of protection, however, this may mean many more years of costly empirical studies. If funders and regulators require these trials to directly compare the efficacy of a new vaccine against RTS,S in 'head-to-head' trials, they will need to be very large indeed. The placebo-controlled phase 3 trial of RTS,S involves 16,000 children11; comparative trials might need to be substantially larger to show superiority.
Any hope of accelerating the evaluation stage of new vaccines thus depends on identifying immune correlates that predict—individually or, more realistically, within the population—whether any particular vaccine candidate will protect against natural infection. Defining correlates of protection requires a much better understanding than we currently have about how these vaccines work, and such correlates will probably be specific for each candidate. Moreover, deployment of RTS,S will necessitate a major re-evaluation of future vaccine needs, taking into consideration operational issues, as well as the benefits and limitations of RTS,S13. This re-evaluation will require a comprehensive and open-minded review of our progress with other vaccines and the parasitological, immunological, methodological and logistical constraints that we face. This review aims to begin this re-evaluation of malaria vaccine research and suggest areas of research that are particularly timely and promising for the immediate future.
RTS,S—the only phase 3 candidate
RTS,S is the first malaria vaccine to approach licensure by entering phase 3 trials. Preliminary data from this trial11 confirmed that the vaccine confers ∼50% protection against clinical malaria episodes, and a similar level of protection against the severe malaria episodes that carry a risk of death, immediately after vaccination in children vaccinated at 5–17 months of age. Even though protection waned within a few months11, and subsequent data from the trial indicated that the vaccine efficacy may be closer to 30% in children vaccinated between 6 and 12 weeks of age12, RTS,S continues on the path to licensure because of its anticipated ability to reduce severe morbidity in young children ( Box 1 ). There are many lessons that we can, and indeed must, learn from the story of the development of RTS,S and questions that we must continue to raise if we hope to improve protection.
Antibodies are not enough. As RTS,S/AS01 has repeatedly protected ∼50% of experimentally challenged volunteers from infection, the immune response of those volunteers has been carefully scrutinized, but there has been little success in identifying a single adaptive immune correlate14. Because of its particulate nature, RTS,S combined with a 3-O-desacyl-4′-monophosphoryl lipid A (MPL)- and QS21-containing depot adjuvant15,16 has generally induced good antibody responses; however, there is a large overlap in antibody titer between protected and unprotected vaccinees17. Thus, whereas a good antibody response to the circumsporozoite protein insert in the vaccine (PfCS) seems to be associated with protection after RTS,S/AS01 vaccination, it may not be sufficient. Over the course of the phase 2 development of RTS,S, there were always protected individuals with low T cell responses and high amounts of antibody and others with good T cell responses but antibody amounts well below the average of the unprotected volunteers17,18,19. This interindividual variation in vaccine responsiveness further complicates the validation of immunological correlates of protection.
RTS,S immune correlates: T cell responses. Detailed phenotypic and functional profiles of antigen-specific T cells induced by RTS,S have not yet been published. Studies have been limited largely to a four-parameter phenotype (CD40L, interferon-γ (IFN-γ), tumor necrosis factor (TNF) and interleukin-2 (IL-2)) identified by the vaccine developers (GlaxoSmithKline) as an indicator of antigen-specific effector T cells20,21,22,23, with additional information from one study in which CD69 and CD25 were assessed as T cell–activation markers24. There are some interesting correlations between protection and numbers of CD4+ T cells producing either TNF or IL-2. RTS,S consistently induces CD4+ but not CD8+ T cell–mediated responses. Whether induction of PfCS-specific CD8+ T cells will improve the efficacy of RTS,S is not known. Future exploration of RTS,S-induced responses should include not only enhanced flow cytometric panels for PfCS-specific effector and memory lymphocytes but also evaluation of the potential for strong adjuvants to induce tolerance, regulatory T cells or both and how their presence or lack thereof correlates with protection.
Optimal vaccination revisited. An understandable desire to integrate RTS,S into existing vaccine schedules, most notably the World Health Organization expanded program of infant immunization, has led to the vast majority of the empiric development being limited to a 0, 1, 2–month vaccination schedule, with licensure being anticipated for the first dose being given at 6–12 weeks of age—although this may be reconsidered in light of the most recent phase 3 trial data12. Although antibody responses at month 10 were similar in children vaccinated on a 0, 1, 2–month and a 0, 1, 7–month schedule and T cell responses were slightly better after the 0, 1, 2–month schedule21, it is not clear that either schedule is optimal for inducing long-lasting responses. Conversely, data from experiments in mice suggest that longer intervals between vaccine doses may provide greater efficacy25,26 and that optimization of vaccination schedules is required for each vaccine candidate. Moreover, in areas of highly seasonal transmission or with extensive maternally acquired immunity, the rationale for a 0, 1, 2–month schedule of infant vaccination is somewhat dubious, especially if protection is short lived. In such a scenario, mass vaccination, annual booster vaccination or both of the population at risk before the annual transmission season might be more effective.
Concerns about potential competition between vaccines in the expanded program of infant immunization coadministered with RTS,S have been allayed in phase 2b trials27 and will be evaluated further within the phase 3 trial. It is not yet clear that either the dose or frequency of RTS,S/AS01 administration has been optimized. There is evidence from other systems that profound stimulation, repeated stimulation or both of antigen-specific T cells may skew the response toward terminally differentiated effector cells at the expense of memory populations28 and that lower doses of antigen may be more effective for the induction of long-lived memory cell populations, self-renewing memory cell populations or both29. The potential impact of previous exposure to vaccine antigens30, resulting from natural infection or in utero exposure, also needs to be considered when optimizing vaccination schedules.
Although considerably more work is required to identify the optimum vaccination age and dosing schedule for RTS,S, more immediate issues are to define an acceptable level of protection and determine the true rate at which vaccine efficacy declines below this level, as this will help determine the optimal boosting strategy. PfCS-specific antibody titers wane rapidly (declining by 80% of their peak after vaccination in 6 months and by 95% within 21 months in one study31), and incidence rates of clinical malaria in vaccinated and unvaccinated individuals begin to converge within 6 months after vaccination31,32,33. Conversely, trials in Mozambique have revealed that vaccinated children have a substantially lower risk than unvaccinated children of developing clinical malaria at up to 45 months after vaccination34 despite the lack of any evidence that anti-PfCS titers in vaccine recipients are boosted by natural exposure35. Various explanations have been put forward to explain this paradox36, but thus far there are no empirical data to support or refute any particular explanation. The planned 3-year follow up of children in the phase 3 trial will begin to provide some answers regarding the duration and mechanisms of protection and how these vary with the local epidemiology of infection.
Given their enormous potential as new adjuvant platforms for vaccines in general, little information has so far been placed in the public domain regarding the innate immune mechanisms induced by the adjuvants AS01 or AS02. A better understanding of the innate pathways activated by these adjuvants would provide invaluable clues as to their probable mode of action and the subsequent effector mechanisms that confer protection, thus substantially assisting future efforts to combine additional antigens or develop rational prime-boost approaches.
Of parasites and immunity: challenges to vaccine development
Ultimately, a sustainable and affordable impact on the disease burden of malaria will require a vaccine efficacy >50%. To achieve this will require a creative synthesis of existing and new strategies. The continued empirical testing of new antigens and formulations remains inevitable, as the potential benefits of a successful trial are so great. The challenge, then, is to use these trials (successful or not) to learn about the underlying immunology and thus guide future improvements.
Malaria's complex life cycle and genetic variability: the major obstacle. The vaccine challenges imposed by the genetically diverse nature of malaria parasite populations and their complex, multistage, multiantigen life cycles have been exhaustively discussed37,38. Evolutionary pressures exerted by the human immune system have selected for extensive polymorphism of genes encoding immunodominant antigens throughout the life cycle39,40. Conversely, conserved genes (or conserved sections of otherwise polymorphic genes, such as those encoding the dominant B cell epitopes of the circumsporozoite protein) are assumed to not have been under substantial immune pressure and, thus, to not be natural targets of protective immunity. The effectiveness of antigenic polymorphism as an immune evasion strategy is amply demonstrated by the observation that homologous re-infection of patients with neurosyphilis undergoing malaria therapy frequently did not induce a clinical infection, whereas re-infection with heterologous strains invariably led to disease41.
The parasite life cycle has evolved, in part, to facilitate rapid selection for, and dissemination of, advantageous mutations. All of the human infective stages are haploid, such that all change-of-function mutations are immediately expressed and able to confer a selective advantage. Extensive within-host replication of blood-stage parasites occurs intracellularly and is thus partially protected from immune recognition and allows for rapid expansion and transmission of parasite clones carrying favorable traits to mosquitoes40. The obligatory sexual stage of the life cycle within the mosquito, combined with the high frequency of mixed-clone infections within a single human and within a single mosquito gut, results in extensive genetic recombination during transmission, bringing together entirely new combinations of polymorphic genes. Indeed, except in rare circumstances of extreme population bottlenecking, every new human malaria infection may be genetically unique and presents a new immunological puzzle40. A final level of antigenic complexity is conferred by the ability of successive generations of parasites to vary the antigens they export to the erythrocyte surface (which mediate the binding of infected erythrocytes to the endothelium in the microcirculation) by sequential expression of individual members of multigenic variant surface protein families; the individual genes encoding these surface antigens are themselves highly polymorphic42,43,44 (Fig. 1).
The immune responses induced by natural infection are therefore highly stage and genotype specific. This might explain the need for a person to experience many different malaria infections before functional immunity (that is, protection from disease) is achieved. Mathematical models suggest that just a single infection reduces the risk of death45 and that immunity to severe malarial complications may develop relatively quickly in areas of intense exposure but can take longer in areas of low or intermittent exposure. There is very little evidence that natural exposure ever leads to complete sterilizing immunity, and most individuals with effective clinical immunity will continue to experience low-density, asymptomatic infections throughout their lives46. It is still unclear whether such broad-spectrum, clinical and partial parasitological immunity is conferred by numerous, highly specific immune responses, by a smaller number of extensively crossreactive immune responses or by the eventual development of immune responses targeting highly conserved proteins. Whether this immunity is a result of specific antiparasite responses that allow individuals to control their parasitaemia or whether it also comprises regulatory or tolerizing responses that control the inflammatory response against the parasite and thereby minimize the clinical symptoms47,48,49 is unknown. The latter concept complicates the development and evaluation of vaccines that currently focus on the induction of proinflammatory, antiparasite effector mechanisms (Fig. 1).
The parasite's life strategy poses other problems for the immune system. Each vertebrate host stage of the parasite is an obligate intracellular pathogen, providing protection from antibody-mediated damage, and the transition from one cell to another is very rapid (sporozoites typically take 10–15 min to migrate from the skin to the liver50, and merozoites invade a red blood cell in less than 30 s51). This offers very little time for antibodies to act. Also, each phase of the life cycle is quite short in relation to the 7–14 d that it takes to develop an effective antigen-specific immune response: sporozoites transform into intrahepatic forms within minutes to hours of entering the body, which then transform into merozoites within 6 d (Fig. 1). Theoretically, a new, antigenically variant generation of merozoites can develop every 48 h. Gametocytes transform into gametes as they emerge from their red-cell cocoon in the mosquito midgut. The immune system is thus always 'running to catch up' with an ever-changing target.
One important exception to the rule that surface proteins are polymorphic is that antigens expressed exclusively on parasite sexual stages developing within the mosquito vector are minimally polymorphic52. This presumably reflects the inability of invertebrates to make highly antigen-specific immune responses; rather, they rely on generic (innate) immune effector mechanisms that target all parasites equally (Fig. 1). The relatively conserved nature of these sexual-stage antigens makes them highly attractive targets for antimalarial vaccines to prevent malaria transmission from mosquitoes to humans.
Malaria's life cycle puzzles the immune system. Although erythrocytic stages induce potent innate immune responses (Fig. 2), sporozoites, immature liver stages and gametocytes induce little, if any, inflammation53. This may indicate that they lack innate immune ligands, that their innate immune ligands are sequestered away from pattern recognition receptors (PRRs) or that there are simply too few of them to induce a potent innate response. Interestingly, viable Plasmodium yoelii liver stages do not induce overt inflammation in BALB/c mice54 but radiation-attenuated parasites do55, suggesting that healthy parasites do indeed sequester ligands away from PRRs. There is also evidence that the parasite may directly subvert the innate response. For example, the circumsporozoite protein interferes with mammalian ribosomal function and host cell–protein synthesis56,57, which might impair the expression of alarm molecules by infected hepatocytes or disrupt their ability to interact in a cognate fashion with adaptive T cells. The rather poor natural immunogenicity of many pre-erythrocytic and sexual-stage antigens may be a direct consequence of this minimal innate immune response. Indeed, vaccination with sporozoite antigens formulated in potent adjuvants such as those used in the RTS,S vaccine induces antibody titers that are at least 20-fold higher than those induced by natural exposure58,59, indicating that the circumsporozoite protein is intrinsically antigenic as long as it is presented in an inflammatory context.
Parasites and parasite-infected red blood cells activate dendritic cells through PRRs, are phagocytosed and their antigens are presented to T cells. PRR signaling leads to the secretion of cytokines that initiate the inflammation that underlies malaria pathogenesis and direct TH1 cell differentiation. TH1 cells provide help for B cell differentiation and antibody secretion and also secrete IFN-γ, which activates macrophages. IFN-γ-activated macrophages phagocytose opsonized parasites and infected red blood cells and subsequently kill them by NO- and O2-dependent pathways. Inflammation induces expression of endothelial adhesion molecules to which infected red blood cells bind. Inflammation is curtailed by the secretion of anti-inflammatory cytokines from macrophages and regulatory populations of T cells. Treg, regulatory T cells; TCR, T cell receptor.
As a vital organ, the liver is programmed to minimize potentially damaging inflammation60, thwarting the priming and recruitment of immune effector cells. Moreover, erythrocytes lack expression of both major histocompatibility complex (MHC) class I and any machinery for the processing and presentation of foreign peptides on their surface, making them resistant to conventional targeting by cytotoxic T cells. Additionally, the enormous biomass of parasite material, much of it in the form of hemozoin (which is highly resistant to biological degradation), that can accumulate during a malaria infection (estimated to be 7 × 1011 P. falciparum–infected red blood cells in an acutely ill adult in southeast Asia61) can overwhelm the degradative capacity of the reticuloendothelial system. Macrophages and dendritic cells become laden with indigestible material and are thus unable to function as effective antigen-presenting cells and are induced to release large amounts of proinflammatory cytokines. This cytokine storm underlies much of the acute pathology of malaria, and immune homeostasis is only restored by activation of regulatory T cell populations, secretion of immunoregulatory cytokines and downregulation of cytotoxic immune mechanisms such as the neutrophil oxidative burst48,62 (Fig. 2).
Naturally acquired immunity to malaria eventually stabilizes at a steady state at which it neither causes excessive immunopathology nor completely eliminates the parasite, resulting in a chronic infection that benefits both the host (that escapes acute disease) and the parasite (that can continue to propagate to new hosts)48. Although arguments can be made in favor of trying to replicate this steady state by vaccination—disease and death would be averted but periodic re-exposure to infection would serve to boost vaccine-induced immunity—designing adjuvants or delivery systems to mimic such a complex interaction is challenging. Moreover, the net result of this steady state is to undermine the host's ability to mount effective immune responses against the malaria parasite itself and against other pathogens, such as Salmonella spp.62 and Epstein-Barr virus63. This is believed to account for much of the indirect burden of malaria. For this reason, if for no other, a better understanding is urgently needed of the interplay of proinflammatory and regulatory immune responses in both natural immunity (Fig. 2) and vaccination models.
Designing the next-generation malaria vaccine
There are sound immunological, epidemiological and clinical arguments for developing vaccines against any, and all, life-cycle stages, and all have been the focus of vaccine development activities64. Although vaccines that directly target gametocytes or gametes (transmission stages) will have the most direct impact on malaria transmission, a vaccine that targets only these stages without preventing infection or ameliorating disease in the vaccinated individual may not be as enthusiastically adopted by governments, health care workers or communities. Moreover, any vaccine that substantially reduces the prevalence and density of circulating asexual parasites may also reduce numbers of infective gametocytes and might therefore contribute to reducing transmission65.
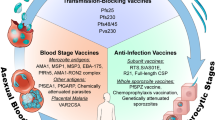
A combination vaccine is the long-term objective of malaria vaccine development, which has historically focused on a very limited set of antigenic targets. Thus far, of the more than 5,000 possible gene candidates, only 20 or so antigens have reached the stage of serious preclinical development64. However, bioinformatic approaches have allowed the identification of additional proteins with favorable vaccine characteristics66, including antigens that are expressed at several life-cycle stages or those that have less allelic variability (Fig. 3).
Pre-erythrocytic vaccines—the ideal candidate. An antisporozoite vaccine that completely blocks the establishment of infection in the liver65,67 would totally prevent clinical disease as well as transmission. Such a vaccine would need to induce high-titer, high-avidity antibodies to trap the sporozoite in the skin before it can invade a blood vessel (and opsonize the sporozoite for uptake and degradation by macrophages) or block essential parasite ligands for hepatocyte-invasion receptors. This ideal vaccine would induce long-lasting antibody responses and memory B cells to protect individuals for life after a single round of immunizations in infancy. Indeed, because vaccinated individuals would not naturally acquire immunity to blood stages and might therefore be highly vulnerable once vaccine immunity waned, these requirements should be seen as prerequisites. This 'holy grail' of malaria vaccine development has remained elusive, although the partially protective RTS,S/AS01 vaccine is a direct result of 50 years of effort towards this goal. New antigen targets for pre-erythrocytic vaccines (to supplement or replace the circumsporozoite protein) are emerging from immunomic approaches68.
The next clear point of attack is the infected hepatocyte. A completely effective vaccine targeting this parasite stage would have the same clinical and epidemiological benefits as a sporozoite-stage vaccine. Despite the difficulty (and risk) of inducing inflammation in the liver, studies in mice have convincingly shown that cytotoxic and cytokine-secreting CD4+ and CD8+ T cells can reduce the number of parasites developing in the liver69 (Fig. 1). These effector cells can be primed by dendritic cells in the lymph nodes70 draining the site of sporozoite inoculation by the mosquito and then migrate to the liver, where they identify infected cells by their presentation of sporozoite antigens on the cell surface71 (Fig. 1). Effector cells can also be induced in situ in the liver: live, radiation-attenuated and genetically attenuated sporozoites that invade liver cells but do not complete their developmental program also induce very potent T cell–mediated immunity in mice72 and primates73, and these T cells seem to be required for protection. It is not yet known which of these two T cell populations (lymph-node primed or liver primed) is most able to confer protection or how dependent the two populations are on inflammation in the liver for their trafficking and survival.
An increasing number of liver stage–specific antigens have been identified74, but diverse attempts to induce protective immunity using protein or virally vectored antigens have so far been disappointing64. Although studies continue on adenovirus-vectored circumsporozoite vaccines, at least 15 other prototype liver-stage vaccines have been abandoned after phase 1 or phase 2 trials64. Many researchers in the field therefore advocate returning to a live, attenuated vaccine approach using irradiated or genetically attenuated parasites as either the immunogen itself or as a tool to identify new liver-stage antigens72,74,75,76. However, potential hurdles to be overcome with attenuated parasites (other than as research tools) include the apparent need to administer them intravenously in some cases77, the possibility that they may revert to virulence or recombine with field isolates to form newly virulent genotypes (as has been recently reported for herpesviruses78) and the technical difficulties associated with the production, standardization, storage and inoculation of live organisms75 (Fig. 3).
Blood-stage vaccines—preventing disease. Vaccines targeting merozoites or infected red blood cells (Fig. 1) should reduce overall parasite densities to levels that do not cause disease. Owing to parasite mutation, replication, antigenic variation and polymorphism, sterile immunity will probably never be achieved by blood-stage vaccination. Thus, erythrocytic vaccines have the potential to reduce disease and death in the short term but may be highly vulnerable to the rapid emergence of parasite escape allelotypes. Three blood-stage vaccine trials have provided evidence for allele-specific protection, with substantial reductions in the numbers of clinical cases caused by parasites carrying vaccine alleles of merozoite surface protein-2 (MSP-2) (ref. 79), apical membrane antigen 1 (AMA-1) (ref. 80) and merozoite surface protein 1 (MSP-1) (E. Angov and C. Ockenhouse, personal communication) but not cases caused by parasites carrying other alleles. It is assumed that both T cells and B cells specific for this stage of the life cycle are induced primarily in the spleen81 (Fig. 2).
So far, clinical trials of blood-stage vaccines have been disappointing: at least 10 candidate vaccines were discontinued at or before phase 2 trials, and only 3 of the 20 remaining candidates have reached a phase 2b study64. Because the handful of merozoite antigens that have been the main focus to date all suffer from high levels of allelic variability, there is increasing interest in the discovery of potential alternatives. Currently, two complementary antigen discovery approaches are being used: first, identifying polymorphic antigens that show population-level signatures of immune selection38, and second, identifying essential, monomorphic or oligomorphic proteins or epitopes that are not naturally immunogenic82. In both approaches, if conserved and functionally constrained epitopes can be identified and their immunogenicity can be enhanced, it might be possible to develop a vaccine that protects against all parasite genotypes and is resilient to immune selection. Combining pre-erythrocytic and blood-stage antigens remains an attractive option to protect recipients of pre-erythrocytic vaccines from breakthrough infections.
Interest in antimerozoite immunity has recently been rekindled by two very exciting, and related, observations. The Ok blood group antigen, basigin, has been identified as an essential receptor for P. falciparum reticulocyte-binding protein homolog 5 (PfRh5), a parasite ligand that is essential for red cell invasion by P. falciparum merozoites83, and PfRh5 has been shown to be a target for vaccine-inducible crossstrain–neutralizing antibodies that prevent merozoite invasion in vitro82. Plasmodium parasites have a diverse and redundant repertoire of invasion pathways, allowing them to adapt to changes in host-cell availability and phenotype, which has frustrated some previous attempts to target merozoite invasion for vaccine development84. But now, for the first time, a universally expressed red cell receptor and an essential, functionally monomorphic, merozoite ligand have been identified. Thus, PfRh5 is an exciting new prospect for erythrocytic vaccine development.
To find other new targets for blood-stage immunity, a much better understanding of the essential immune effector mechanisms that control blood-stage infections is needed (Fig. 1). Although there is good evidence that antibodies—acting alone or in concert with phagocytes—can remove infected red blood cells from the circulation85,86 and are associated with protection87, the antigens targeted by functionally important antibodies are still poorly characterized, as is their precise mechanism of action, and they are often highly allele specific. Furthermore, merozoite antigen–specific T helper type 1 (TH1) and TH2 cells can be detected in immune88,89 and immunized90,91 individuals, but their importance is unclear. Studies of primary malaria infections in mice indicate that natural killer (NK) cells, T cells and B cells are all required for the effective control and clearance of blood-stage parasites92 (Fig. 2). These cells probably mediate their effects sequentially during infection: NK cells, gd T cells and phagocytes might restrain the earliest phases of parasite growth, T cells may help limit the peak of parasitemia, and antibodies might 'mop up' the residual infection, preventing recrudescence93. NK cell– and T cell–derived IFN-γ is assumed to enhance the phagocytosis of infected red blood cells by macrophages. However, one important gap in our knowledge is whether the immune mechanisms that control primary infections are the same as those that control secondary and subsequent malaria infections. For example, during re-infection, antibodies of appropriate specificity might act to immediately contain the infection with a reduced requirement for a cell-mediated component. As both mice and nonhuman primates are more or less resistant to re-infection with many commonly used Plasmodium spp. and strains, there are few good animal models for secondary and subsequent infections. Therefore, the relative contribution of cell-mediated and antibody-mediated effector mechanisms to immunity to re-infection is very difficult to establish.
Supporting a role for TH1 effector cells in immunity to asexual blood stages, a study using repeated ultra-low doses of unattenuated infected red blood cells followed by drug treatment before the onset of clinical symptoms induced sterile protection against homologous challenge in three of four previously malaria-naive subjects. Immunization induced IFN-γ–producing CD4+ and CD8+ T cells, but no antibodies to parasites or infected red blood cells were detected94. Roestenberg and colleagues95 adopted a similar approach using repeated infected mosquito bites with concurrent chloroquine prophylaxis as their immunization strategy. All ten immunized subjects were protected against homologous challenge infection (and four out of six were protected when challenged again 28 months after immunization96), and CD4+ T cells producing IFN-γ, TNF and IL-2 after in vitro re-stimulation with both sporozoites and erythrocytic parasites were identified as potential markers of protection. Whether liver-stage or blood-stage antigens are the primary targets of these T cells is not yet known, but data from a mouse model system indicate that late-stage liver parasites can express blood-stage antigens and induce protective immunity against challenge with blood stages, as well as with sporozoites97. In mice, immunization with late liver stage–arresting parasites can confer cross-species immunity97, but, to our knowledge, neither the sporozoite nor the merozoite infection and chemoprophylaxis approach has yet been shown to confer protection against heterologous challenge, which will be essential for the vaccines to be efficacious in field settings. Indeed, epidemiologists were initially somewhat skeptical about the real impact of these observations given that many people in endemic countries who are infected with malaria are also receiving antimalarial drugs. Yet the sustained protection from malaria associated in some studies with sulfadoxine-pyrimethamine treatment—either as intermittent preventative therapy or for parasite clearance in vaccine trials—suggests that substantially reducing the density of blood-stage infections or disrupting parasite development in the liver may allow more effective development of blood-stage immunity36,98. Recent experimental evidence strongly supports the latter mechanism: in addition to the studies with late liver stage–arresting parasites cited above97, drugs that inhibit the development of Plasmodium liver stages enhance the development of naturally acquired immunity in rodent malaria models98.
Another promising avenue of blood-stage vaccine research is the quest for a vaccine that will induce antibodies that prevent sequestration of infected red blood cells in the placenta. Placental sequestration is mediated by the binding of particular PfEMP-1 variants to complex polysaccharides (primarily chondroitin sulfate A (CSA))99. CSA binding seems to be a property of a rather small subset of the PfEMP-1 repertoire, raising hopes that an oligovalent vaccine may be able to raise antibodies that will block most placental sequestration99. The repertoire of PfEMP-1 variants mediating sequestration elsewhere in the body is much broader, making the prospect of a vaccine to prevent other manifestations of severe malaria much less probable. Yet as discrete clusters of PfEMP-1 variants are identified that preferentially bind to particular receptors100, the molecular signatures of receptor binding might be identified and allow for the targeting of specific clinical syndromes by vaccination.
Transmission-blocking vaccines—at the root of eradication. Targeting sexual stages of the life cycle (gametocytes, gametes and ookinetes) to prevent the development of infectious sporozoites within the mosquito (Fig. 1) is the vaccine approach most aligned with the current malaria eradication and elimination agenda. Although such vaccines could provide 'herd immunity' to reduce transmission, they would not confer individual protection from infection or disease; this may make the regulatory processes more complex65 and may influence the perceived cost-benefit analyses made by national governments, as well as the acceptability of the vaccine at the individual or community level.
The surface proteins of these life-cycle stages are much less polymorphic than their asexual-stage counterparts and are therefore more amenable to vaccine development. Transmission-blocking immunity seems to be mediated entirely by the humoral elements of antibody and complement, and preclinical vaccine testing is facilitated by the availability of a realistic bioassay101,102. Indeed, a transmission-blocking vaccine might be licensed on the basis of safety and immunogenicity data without the need for large randomized trials, but if clinical-efficacy trials are deemed necessary, cluster-randomized trials of entire villages in several different transmission settings will probably be required. Another (probably surmountable) hurdle is that gamete and gametocyte surface proteins have complex secondary and tertiary structures with numerous disulphide bonds, which have made them challenging to express in their native form and resulted in vaccine candidates that induce antibody with poor binding to the native protein101. A final hurdle is that several antigens expressed during mosquito infection are not expressed in humans, and therefore antibodies raised by vaccination are unlikely to be boosted by subsequent natural infection.
Inducing and maintaining effector responses
For reasons still unknown, the induction of robust, appropriate and durable immune effector responses to many malaria antigens is highly problematic103. The identification of appropriate effector responses has been very difficult, even when protective efficacy can be documented, as every combination of antigen and delivery platform seems to induce a different pattern of responses. These responses vary greatly even among recipients of a single vaccine, and there is currently no consistent, predictive relationship between induction of any particular response and protection17.
Identifying appropriate effector responses—what are we looking for and how do we find it? Cellular immunity to malaria is extraordinarily complex (Fig. 2). Acute, proinflammatory, cytokine-mediated effector responses from both the innate and effector arms of the immune system can limit the initial replication phase of blood-stage parasites and reduce direct damage to the host, such as hemolysis and erythrocyte degradation92,93. But these responses need to be quickly controlled by anti-inflammatory mechanisms to prevent immunopathology, notably by IL-10–secreting CD4+ T cells48 (Fig. 2). Eventual control, clearance or both of parasitemia, however, depends on antibody-mediated responses, which become more effective over time, presumably because of the gradual acquisition and affinity maturation of antibodies recognizing prevalent parasite genotypes. Antibody responses also seem highly relevant for tackling other parasite life-cycle stages, but we face considerable challenges in generating antimalarial antibodies in sufficient quantity and quality and keeping them at high enough levels to provide long-term protection. This will probably only be resolved when we have a better understanding of the precise role that TH cells have in shaping antimalarial B cell responses and B cell memory, as well as the factors that are important for the generation of long-lived plasma cells.
Virally vectored vaccines are more efficient at inducing antigen-specific IFN-γ–secreting T cells compared to most adjuvanted protein vaccines, but the magnitude of these responses does not correlate with vaccine efficacy104,105, even when the vaccines are given in heterologous combination to ensure boosting of malaria-specific rather than vector-specific responses106,107,108. Effective immunization may therefore depend at least as much on key qualitative parameters of T cell function as it does on absolute numbers of antigen-specific T cells. The unremitting focus on peptide-specific IFN-γ secretion as a measure of T cell responsiveness109,110 has only very recently given way to the consideration of a wider array of T cell functions, but even so, as evidenced in the recent RTS,S studies20,21,22,23,24, the number of markers being studied is still very limited. The importance of T cell trafficking to target tissues, and the role of specific chemokines and chemokine receptors in this process, has begun to be investigated in mouse models of malarial pathology111,112, but chemokine receptor expression is only just beginning to be examined in effector T cell populations among vaccinated individuals113. Clearly we now need to take a broader look at potential markers of an effective T cell response. Genome-wide transcriptional analysis of vaccine responses, such as those recently applied to yellow fever114 and influenza115 vaccines and those currently underway for HIV vaccines116, may provide some insight into potential correlates of immune protection.
However, to determine which responses are the true mediators of protection and which are simply nonspecific (for example, adjuvant-induced) epiphenomena, we must differentiate the vaccinated individuals who are truly immune from similarly vaccinated individuals who are not. As immunization with multiple doses of irradiated sporozoites remains the most reliable means of inducing sterilizing immunity against challenge infection117, a concerted effort is needed to characterize the immunological correlates of irradiated sporozoite-induced immunity in humans and to follow up protected individuals until that immunity is lost. Similarly, the very large phase 3 trial of the RTS,S vaccine that is currently underway11 provides an opportunity to identify humoral correlates, cellular correlates or both of protection conferred by this vaccine. We suggest that multiparameter flow cytometric and transcriptional analyses of antigen-specific T cells from RTS,S-vaccinated individuals should be given high priority. Furthermore, these responses need to be monitored over time after vaccination to determine the longevity of the memory cell population. Ideally, this would be combined with repeated in vitro assays to assess the speed of differentiation of memory cells into effector cells after re-exposure to antigen, as well as the ability of memory cells to self renew after antigen challenge. A similar analysis of B cell responses might also be informative.
Despite the power of the genome-wide transcriptional approach, teasing out protective antigen-specific immune responses from neutral or even harmful responses, either in naturally infected populations or vaccine recipients, is complicated by the inevitably highly polyclonal nature of the response. For example, the host can develop antibody responses to some epitopes of MSP-1 that prevent invasion in vitro but can also develop so-called 'blocking' antibodies to other epitopes on the same molecule that prevent those helpful antibodies from binding, permitting the parasite to effectively invade once again118. A better understanding of the fine specificity of antibody responses to other potential vaccine candidates is greatly needed. In naturally infected individuals, protective antibodies may be induced with the same frequency and kinetics as are functionally irrelevant or detrimental antibodies119, making it impossible to determine which, if any, of the antibodies in a given serum sample might be mediating an antiparasitic effect. The use of genetically modified parasites that do not express particular antigens, such as PfEMP-1 (ref. 120), or express orthologous but noncrossreactive antigens from other malarial species, such as MSP-1 (ref. 121), has allowed the dissection of humoral responses to some blood-stage antigens, and this approach deserves further attention.
Maintaining long-lived responses—how to hang on to what we've got. Animal studies have shown that maintaining high amounts of circulating antibodies, presumably by generating large numbers of long-lived plasma cells, is important for antisporozoite vaccines122. The transient nature of this stage of the life cycle precludes any possibility that reactivation of sporozoite-specific memory B cells and their differentiation into antibody-secreting cells would provide adequate protection, except in environments where persistent high levels of sporozoite exposure might allow antibodies to be maintained by successive generations of short-lived plasma cells. However, there is evidence that B cell exhaustion may become a problem in such circumstances123. Maintaining high amounts of antisporozoite antibodies has been, and remains, a major challenge for sporozoite vaccination and will probably only be overcome by identifying better adjuvants or delivery systems.
Conversely, although it may be highly desirable to maintain high amounts of effectors against merozoites and gametocytes, boosting of antibody responses from memory B cells—and/or conversion of central memory T cells into effector cells—may be a viable option for these life-cycle stages. Merozoite antigens begin to be expressed, albeit at quite low levels, within the first 2 d of the 7-d differentiation of intrahepatic parasites124, and merozoite numbers increase steadily over the first few days after their emergence from the liver such that the parasite densities that cause clinical disease are typically not reached until 9 or more days after sporozoite inoculation125. This is sufficient time for substantial boosting of antibody responses from memory B cells or for differentiation of effector T cells from memory cells. Similarly, some gametocyte antigens begin to be expressed very early in the process of gametocytogenesis, and gametocytes may be found in the circulation for many weeks after the initial infection126, providing ample time for antibody titers to be boosted. In practice, however, gametocyte-specific antibodies are often present at only very low titers and may not be boosted by infection127; the reasons for this are not understood.
Where next for malaria vaccines?
RTS,S may soon be licensed and deployed and, in combination with intensive vector control and improved management of clinical malaria cases, will help to maintain the momentum toward malaria control. At only 30-50% efficacy, RTS,S will not in itself be a tool for malaria elimination or eradication. Sustainable and affordable control of malaria in the long term requires a highly effective vaccine that induces life-long immunity.
In our opinion, the spectrum of routes to this goal encompasses two extremes. The first is to continue trying to improve the efficacy of the prototype vaccines and candidate antigens that we already have, most of which have been under development for close to 20 years. The long and complicated story of RTS,S development is a clear validation of this approach, but the costs involved are such that very few candidates can be taken forward into the next generation of vaccines, and there is a need, regrettably, to 'down select' many potentially promising avenues of research. The second route is to go back to the drawing board and use everything we have learned and can learn about the biology of the parasite and the immune response of the host to develop entirely new approaches ( Box 2 ). Both routes will be expensive, neither is guaranteed to work, and the most probable path forward will involve both. Moreover, demonstrating the efficacy of any new vaccine may raise ethical, financial and licensing dilemmas. Will a new vaccine have to show superiority to RTS,S in head-to-head clinical trials? For ethical reasons, will it have to be given in combination with RTS,S? How big (and therefore, how expensive) will such trials be?
Mathematical modeling of immune parameters and clinical outcomes of large-scale vaccine studies offers a relatively inexpensive means for prioritizing future research. For example, the benefits of combining antigens from different life-cycle stages into a polyvalent vaccine could be assessed, the extent to which vaccine efficacy is compromised by the genetic diversity of the parasite or host population could be estimated, and the impact on the trial outcome of using different clinical or parasitological endpoints could be compared. The balance between intended and unintended consequences of choices made during antigen, delivery-platform and immunization-schedule optimization needs to be more clearly evaluated and understood. It is increasingly clear that all three of these variables contribute to the induction of an immune response; a formulation optimized for one antigen cannot be assumed to be optimal for another, and the optimal interval between doses will vary depending on the antigen, the platform and the desired immune response. Increasingly complex delivery platforms, such as viral vectors and heterologous prime-boost combinations, may need to be considered to generate the kind of enhanced protection that will represent a true step forward.
In reality, malaria vaccine development will continue to comprise incremental advances, coming from empirical approaches as well as fundamental research. We need to ensure, however, that these approaches are well integrated. It is essential that the biological materials and data generated by both approaches are subjected to detailed scrutiny so that we can begin to understand, for example, how different adjuvants and vaccine dosing schedules affect the balance and durability of effector, memory and regulatory responses. The raw immunological and epidemiological data from clinical trials need to be made publicly available so that they can be widely interrogated and used and so that lessons learned from vaccine research for any infectious disease can be immediately applied to malaria vaccines and vice versa. It is only through such a holistic approach that we will derive maximum benefit from all our activities and give ourselves the best chance of solving this highly intractable problem.
References
World Health Organization. World malaria report 2011. World Health Organization. <http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng.pdf> (2011).
Murray, C.J. et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379, 413–431 (2012).
Umbers, A.J., Aitken, E.H. & Rogerson, S.J. Malaria in pregnancy: small babies, big problem. Trends Parasitol. 27, 168–175 (2011).
Cox-Singh, J. et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46, 165–171 (2008).
O'Brien, C., Henrich, P.P., Passi, N. & Fidock, D.A. Recent clinical and molecular insights into emerging artemisinin resistance in Plasmodium falciparum. Curr. Opin. Infect. Dis. 24, 570–577 (2011).
McNamara, C. & Winzeler, E.A. Target identification and validation of novel antimalarials. Future Microbiol. 6, 693–704 (2011).
Ranson, H. et al. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 27, 91–98 (2011).
Bousema, T. & Drakeley, C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 24, 377–410 (2011).
Malaria Vaccine Technology Roadmap. Malaria vaccine technology roadmap. PATH Malaria Vaccine Initiative. <http://www.malariavaccine.org/files/Malaria_Vaccine_TRM_Final_000.pdf> (2006).
Bill and Melinda Gates Foundation. Malaria strategy overview. Bill and Melinda Gates Foundation. <http://www.gatesfoundation.org/malaria/Documents/malaria-strategy.pdf> (2011).
Agnandji, S.T. et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365, 1863–1875 (2011).
RTS,S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367, 2284–2295 (2012).
Alonso, P.L. & Tanner, M. Public health challenges and prospects for malaria control and elimination. Nat. Med. 19, 150–155 (2013).
Casares, S., Brumeanu, T.D. & Richie, T.L. The RTS,S malaria vaccine. Vaccine 28, 4880–4894 (2010).
Garçon, N. & Van Mechelen, M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev. Vaccines 10, 471–486 (2011).
Regules, J.A., Cummings, J.F. & Ockenhouse, C.F. The RTS,S vaccine candidate for malaria. Expert Rev. Vaccines 10, 589–599 (2011).
Kester, K.E. et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 200, 337–346 (2009).
Stoute, J.A. et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Med. 336, 86–91 (1997).
Stoute, J.A. et al. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J. Infect. Dis. 178, 1139–1144 (1998).
Olotu, A. et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria. PLoS ONE 6, e25786 (2011).
Ansong, D. et al. T cell responses to the RTS,S/AS01(E) and RTS,S/AS02(D) malaria candidate vaccines administered according to different schedules to Ghanaian children. PLoS ONE 6, e18891 (2011).
Lumsden, J.M. et al. Protective immunity induced with the RTS,S/AS vaccine is associated with IL-2 and TNF-α producing effector and central memory CD4 T cells. PLoS ONE 6, e20775 (2011).
Agnandji, S.T. et al. Induction of Plasmodium falciparum–specific CD4+ T cells and memory B cells in Gabonese children vaccinated with RTS,S/AS01(E) and RTS,S/AS02(D). PLoS ONE 6, e18559 (2011).
Horowitz, A. et al. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J. Immunol. 188, 5054–5062 (2012).
Brice, G.T. et al. Extended immunization intervals enhance the immunogenicity and protective efficacy of plasmid DNA vaccines. Microbes Infect. 9, 1439–1446 (2007).
Elnekave, M., Bivas-Benita, M., Gillard, G.O., Sircar, P. & Hovav, A.H. A matter of timing: unsynchronized antigen expression and antigen presentation diminish secondary T cell responses. J. Immunol. 183, 1013–1021 (2009).
Abdulla, S. et al. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N. Engl. J. Med. 359, 2533–2544 (2008).
Obar, J.J. & Lefrancois, L. Early signals during CD8 T cell priming regulate the generation of central memory cells. J. Immunol. 185, 263–272 (2010).
Fousteri, G. et al. Increased memory conversion of naive CD8 T cells activated during late phases of acute virus infection due to decreased cumulative antigen exposure. PLoS ONE 6, e14502 (2011).
Croom, H.A. et al. Memory precursor phenotype of CD8+ T cells reflects early antigenic experience rather than memory numbers in a model of localized acute influenza infection. Eur. J. Immunol. 41, 682–693 (2011).
Alonso, P.L. et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366, 2012–2018 (2005).
Alonso, P.L. et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364, 1411–1420 (2004).
Bejon, P. et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 359, 2521–2532 (2008).
Sacarlal, J. et al. Long-term safety and efficacy of the RTS,S/AS02A malaria vaccine in Mozambican children. J. Infect. Dis. 200, 329–336 (2009).
Aide, P. et al. Four year immunogenicity of the RTS,S/AS02(A) malaria vaccine in Mozambican children during a phase IIb trial. Vaccine 29, 6059–6067 (2011).
Sutherland, C.J., Drakeley, C.J. & Schellenberg, D. How is childhood development of immunity to Plasmodium falciparum enhanced by certain antimalarial interventions? Malar. J. 6, 161 (2007).
Takala, S.L. & Plowe, C.V. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming 'vaccine resistant malaria'. Parasite Immunol. 31, 560–573 (2009).
Weedall, G.D. & Conway, D.J. Detecting signatures of balancing selection to identify targets of anti-parasite immunity. Trends Parasitol. 26, 363–369 (2010).
Epstein, J.E., Giersing, B., Mullen, G., Moorthy, V. & Richie, T.L. Malaria vaccines: are we getting closer? Curr. Opin. Mol. Ther. 9, 12–24 (2007).
Mackinnon, M.J. & Marsh, K. The selection landscape of malaria parasites. Science 328, 866–871 (2010).
McKenzie, F.E., Smith, D.L., O'Meara, W.P. & Riley, E.M. Strain theory of malaria: the first 50 years. Adv. Parasitol. 66, 1–46 (2008).
Dzikowski, R. & Deitsch, K.W. Genetics of antigenic variation in Plasmodium falciparum. Curr. Genet. 55, 103–110 (2009).
Pasternak, N.D. & Dzikowski, R. PfEMP1: an antigen that plays a key role in the pathogenicity and immune evasion of the malaria parasite Plasmodium falciparum. Int. J. Biochem. Cell Biol. 41, 1463–1466 (2009).
Chen, D.S. et al. A molecular epidemiological study of var gene diversity to characterize the reservoir of Plasmodium falciparum in humans in Africa. PLoS ONE 6, e16629 (2011).
Gupta, S., Snow, R.W., Donnelly, C.A., Marsh, K. & Newbold, C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5, 340–343 (1999).
Okell, L.C., Ghani, A.C., Lyons, E. & Drakeley, C.J. Submicroscopic infection in Plasmodium falciparum–endemic populations: a systematic review and meta-analysis. J. Infect. Dis. 200, 1509–1517 (2009).
Riley, E.M., Wahl, S., Perkins, D.J. & Schofield, L. Regulating immunity to malaria. Parasite Immunol. 28, 35–49 (2006).
Finney, O.C., Riley, E.M. & Walther, M. Regulatory T cells in malaria—friend or foe? Trends Immunol. 31, 63–70 (2010).
Hansen, D.S. & Schofield, L. Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog. 6, e1000771 (2010).
Sidjanski, S. & Vanderberg, J.P. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am. J. Trop. Med. Hyg. 57, 426–429 (1997).
Gilson, P.R. & Crabb, B.S. Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int. J. Parasitol. 39, 91–96 (2009).
Niederwieser, I., Felger, I. & Beck, H.P. Limited polymorphism in Plasmodium falciparum sexual-stage antigens. Am. J. Trop. Med. Hyg. 64, 9–11 (2001).
Liehl, P. & Mota, M.M. Innate recognition of malarial parasites by mammalian hosts. Int. J. Parasitol. 42, 557–566 (2012).
Vanderberg, J.P., Khan, Z.M. & Stewart, M.J. Induction of hepatic inflammatory response by Plasmodium berghei sporozoites protects BALB/c mice against challenge with Plasmodium yoelii sporozoites. J. Parasitol. 79, 763–767 (1993).
Khan, Z.M. & Vanderberg, J.P. Specific inflammatory cell infiltration of hepatic schizonts in BALB/c mice immunized with attenuated Plasmodium yoelii sporozoites. Int. Immunol. 4, 711–718 (1992).
Frevert, U. et al. Malaria circumsporozoite protein inhibits protein synthesis in mammalian cells. EMBO J. 17, 3816–3826 (1998).
Hügel, F.U., Pradel, G. & Frevert, U. Release of malaria circumsporozoite protein into the host cell cytoplasm and interaction with ribosomes. Mol. Biochem. Parasitol. 81, 151–170 (1996).
Bojang, K.A. et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358, 1927–1934 (2001).
Polhemus, M.E. et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS ONE 4, e6465 (2009).
Crispe, I.N. et al. Cellular and molecular mechanisms of liver tolerance. Immunol. Rev. 213, 101–118 (2006).
Dondorp, A.M. et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2, e204 (2005).
Cunnington, A.J., de Souza, J.B., Walther, M. & Riley, E.M. Malaria impairs resistance to Salmonella through heme- and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat. Med. 18, 120–127 (2012).
Moormann, A.M., Snider, C.J. & Chelimo, K. The company malaria keeps: how co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Curr. Opin. Infect. Dis. 24, 435–441 (2011).
Schwartz, L., Brown, G.V., Genton, B. & Moorthy, V.S. A review of malaria vaccine clinical projects based on the WHO rainbow table. Malar. J. 11, 11 (2012).
The malERA Consultative Group on Vaccines. A research agenda for malaria eradication: vaccines. PLoS Med. 8, e1000398 (2011).
Doolan, D.L. Plasmodium immunomics. Int. J. Parasitol. 41, 3–20 (2011).
Plowe, C.V., Alonso, P. & Hoffman, S.L. The potential role of vaccines in the elimination of falciparum malaria and the eventual eradication of malaria. J. Infect. Dis. 200, 1646–1649 (2009).
Matuschewski, K., Hafalla, J.C., Borrmann, S. & Friesen, J. Arrested Plasmodium liver stages as experimental anti-malaria vaccines. Hum. Vaccin. (suppl. 7), 16–21 (2011).
Cockburn, I.A. & Zavala, F. T cell memory in malaria. Curr. Opin. Immunol. 19, 424–429 (2007).
Chakravarty, S. et al. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 13, 1035–1041 (2007).
Cockburn, I.A. et al. Dendritic cells and hepatocytes use distinct pathways to process protective antigen from Plasmodium in vivo. PLoS Pathog. 7, e1001318 (2011).
Matuschewski, K., Hafalla, J.C., Borrmann, S. & Friesen, J. Arrested Plasmodium liver stages as experimental anti-malaria vaccines. Hum. Vaccin. (suppl. 7), 16–21 (2011).
Weiss, W.R. & Jiang, C.G. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS ONE 7, e31247 (2012).
Lindner, S.E., Miller, J.L. & Kappe, S.H. Malaria parasite pre-erythrocytic infection: preparation meets opportunity. Cell. Microbiol. 14, 316–324 (2012).
Hoffman, S.L. et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum. Vaccin. 6, 97–106 (2010).
Vaughan, A.M., Wang, R. & Kappe, S.H. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum. Vaccin. 6, 107–113 (2010).
Epstein, J.E. et al. Live attenuated malaria vaccine designed to protect through hepatic CD8 T cell immunity. Science 334, 475–480 (2011).
Lee, S.W. et al. Attenuated vaccines can recombine to form virulent field viruses. Science 337, 188 (2012).
Genton, B. et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1–2b trial in Papua New Guinea. J. Infect. Dis. 185, 820–827 (2002).
Thera, M.A. et al. A field trial to assess a blood-stage malaria vaccine. N. Engl. J. Med. 365, 1004–1013 (2011).
Engwerda, C.R., Beattie, L. & Amante, F.H. The importance of the spleen in malaria. Trends Parasitol. 21, 75–80 (2005).
Douglas, A.D. et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat. Commun. 2, 601 (2011).
Crosnier, C. et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480, 534–537 (2011).
Tham, W.H., Healer, J. & Cowman, A.F. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol. 28, 23–30 (2012).
Cohen, S., Mc, G.I. & Carrington, S. γ-globulin and acquired immunity to human malaria. Nature 192, 733–737 (1961).
Sabchareon, A. et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45, 297–308 (1991).
Fowkes, F.J., Richards, J.S., Simpson, J.A. & Beeson, J.G. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 7, e1000218 (2010).
Riley, E.M., Jepsen, S., Andersson, G., Otoo, L.N. & Greenwood, B.M. Cell-mediated immune responses to Plasmodium falciparum antigens in adult Gambians. Clin. Exp. Immunol. 71, 377–382 (1988).
Troye-Blomberg, M. et al. Production by activated human T cells of interleukin 4 but not interferon-γ is associated with elevated levels of serum antibodies to activating malaria antigens. Proc. Natl. Acad. Sci. USA 87, 5484–5488 (1990).
Teirlinck, A.C. et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog. 7, e1002389 (2011).
Huaman, M.C. et al. Ex vivo cytokine and memory T cell responses to the 42-kDa fragment of Plasmodium falciparum merozoite surface protein-1 in vaccinated volunteers. J. Immunol. 180, 1451–1461 (2008).
Stevenson, M.M. & Riley, E.M. Innate immunity to malaria. Nat. Rev. Immunol. 4, 169–180 (2004).
Langhorne, J., Ndungu, F.M., Sponaas, A.M. & Marsh, K. Immunity to malaria: more questions than answers. Nat. Immunol. 9, 725–732 (2008).
Pombo, D.J. et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360, 610–617 (2002).
Roestenberg, M. et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361, 468–477 (2009).
Roestenberg, M. et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377, 1770–1776 (2011).
Butler, N.S. et al. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9, 451–462 (2011).
Friesen, J. et al. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci. Transl. Med. 2, 40ra49 (2010).
Hviid, L. The role of Plasmodium falciparum variant surface antigens in protective immunity and vaccine development. Hum. Vaccin. 6, 84–89 (2010).
Janes, J.H. et al. Investigating the host binding signature on the Plasmodium falciparum PfEMP1 protein family. PLoS Pathog. 7, e1002032 (2011).
Saul, A. Mosquito stage, transmission blocking vaccines for malaria. Curr. Opin. Infect. Dis. 20, 476–481 (2007).
van der Kolk, M. et al. Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission-reducing activity using empirical data. Parasitology 130, 13–22 (2005).
Todryk, S.M. et al. Multiple functions of human T cells generated by experimental malaria challenge. Eur. J. Immunol. 39, 3042–3051 (2009).
Reyes-Sandoval, A. et al. CD8+ T effector memory cells protect against liver-stage malaria. J. Immunol. 187, 1347–1357 (2011).
Tamminga, C. et al. Adenovirus-5–vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component. PLoS ONE 6, e25868 (2011).
Forbes, E.K. et al. Combining liver- and blood-stage malaria viral-vectored vaccines: investigating mechanisms of CD8+ T cell interference. J. Immunol. 187, 3738–3750 (2011).
Reyes-Sandoval, A. et al. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect. Immun. 78, 145–153 (2010).
Stewart, V.A. et al. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect. Immun. 75, 2283–2290 (2007).
Vuola, J.M. et al. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J. Immunol. 174, 449–455 (2005).
Bejon, P. et al. Immunogenicity of the candidate malaria vaccines FP9 and modified vaccinia virus Ankara encoding the pre-erythrocytic antigen ME-TRAP in 1–6 year old children in a malaria endemic area. Vaccine 24, 4709–4715 (2006).
Nie, C.Q. et al. IP-10–mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog. 5, e1000369 (2009).
Van den Steen, P.E. et al. CXCR3 determines strain susceptibility to murine cerebral malaria by mediating T lymphocyte migration toward IFN-γ–induced chemokines. Eur. J. Immunol. 38, 1082–1095 (2008).
Berthoud, T.K., Dunachie, S.J., Todryk, S., Hill, A.V. & Fletcher, H.A. MIG (CXCL9) is a more sensitive measure than IFN-γ of vaccine induced T-cell responses in volunteers receiving investigated malaria vaccines. J. Immunol. Methods 340, 33–41 (2009).
Querec, T.D. et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10, 116–125 (2009).
Nakaya, H.I. et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 12, 786–795 (2011).
Andersen-Nissen, E., Heit, A. & McElrath, M.J. Profiling immunity to HIV vaccines with systems biology. Curr. Opin. HIV AIDS 7, 32–37 (2012).
Luke, T.C. & Hoffman, S.L. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J. Exp. Biol. 206, 3803–3808 (2003).
Uthaipibull, C. et al. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307, 1381–1394 (2001).
Nwuba, R.I. et al. The human immune response to Plasmodium falciparum includes both antibodies that inhibit merozoite surface protein 1 secondary processing and blocking antibodies. Infect. Immun. 70, 5328–5331 (2002).
Voss, T.S. et al. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature 439, 1004–1008 (2006).
O'Donnell, R.A., Saul, A., Cowman, A.F. & Crabb, B.S. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat. Med. 6, 91–95 (2000).
Doolan, D.L., Dobano, C. & Baird, J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 22, 13–36 (2009).
Weiss, G.E. et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J. Immunol. 183, 2176–2182 (2009).
Bodescot, M. et al. Transcription status of vaccine candidate genes of Plasmodium falciparum during the hepatic phase of its life cycle. Parasitol. Res. 92, 449–452 (2004).
Epstein, J.E. et al. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum–infected mosquitoes: an update. J. Infect. Dis. 196, 145–154 (2007).
Bousema, T. et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 9, 136 (2010).
Bousema, T. et al. The dynamics of naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs230 & Pfs48/45 in a low endemic area in Tanzania. PLoS ONE 5, e14114 (2010).
Bojang, K.A. RTS,S/AS02A for malaria. Expert Rev. Vaccines 5, 611–615 (2006).
Nussenzweig, V. & Nussenzweig, R.S. Rationale for the development of an engineered sporozoite malaria vaccine. Adv. Immunol. 45, 283–334 (1989).
Heppner, D.G. & Ballou, W.R. Malaria in 1998: advances in diagnosis, drugs and vaccine development. Curr. Opin. Infect. Dis. 11, 519–530 (1998).
Ballou, W.R. The development of the RTS,S malaria vaccine candidate: challenges and lessons. Parasite Immunol. 31, 492–500 (2009).
Acknowledgements
We thank all the mentors, colleagues, students, funders and volunteers whose support, hard work, ideas and discussions have contributed to our thinking over many years. The material in this article does not reflect the endorsement, official attitude or position of the London School of Hygiene and Tropical Medicine, the Uniformed Services University of the Health Sciences or the US Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Riley, E., Stewart, V. Immune mechanisms in malaria: new insights in vaccine development. Nat Med 19, 168–178 (2013). https://doi.org/10.1038/nm.3083
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3083
This article is cited by
-
Association of apoptosis-related variants to malaria infection and parasite density in individuals from the Brazilian Amazon
Malaria Journal (2023)
-
Evaluation of the Pfs25-IMX313/Matrix-M malaria transmission-blocking candidate vaccine in endemic settings
Malaria Journal (2022)
-
Genetic diversity of Plasmodium falciparum AMA-1 antigen from the Northeast Indian state of Tripura and comparison with global sequences: implications for vaccine development
Malaria Journal (2022)
-
Plants as Sources of Natural and Recombinant Antimalaria Agents
Molecular Biotechnology (2022)
-
Subolesin vaccination inhibits blood feeding and reproduction of Haemaphysalis longicornis in rabbits
Parasites & Vectors (2020)