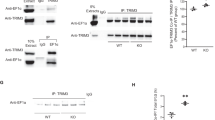
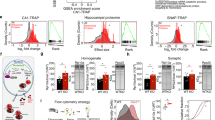
Abstract
Fragile X syndrome (FXS), the most common cause of inherited mental retardation and autism, is caused by transcriptional silencing of FMR1, which encodes the translational repressor fragile X mental retardation protein (FMRP). FMRP and cytoplasmic polyadenylation element–binding protein (CPEB), an activator of translation, are present in neuronal dendrites, are predicted to bind many of the same mRNAs and may mediate a translational homeostasis that, when imbalanced, results in FXS. Consistent with this possibility, Fmr1−/y; Cpeb1−/− double-knockout mice displayed amelioration of biochemical, morphological, electrophysiological and behavioral phenotypes associated with FXS. Acute depletion of CPEB1 in the hippocampus of adult Fmr1−/y mice rescued working memory deficits, demonstrating reversal of this FXS phenotype. Finally, we find that FMRP and CPEB1 balance translation at the level of polypeptide elongation. Our results suggest that disruption of translational homeostasis is causal for FXS and that the maintenance of this homeostasis by FMRP and CPEB1 is necessary for normal neurologic function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Penagarikano, O., Mulle, J.G. & Warren, S.T. Annu. Rev. Genomics Hum. Genet. 8, 109–129 (2007).
Santoro, M.R., Bray, S.M. & Warren, S.T. Annu. Rev. Pathol. 7, 219–245 (2012).
Verkerk, A.J. et al. Cell 65, 905–914 (1991).
Feng, Y. et al. J. Neurosci. 17, 1539–1547 (1997).
Darnell, J.C. et al. Cell 146, 247–261 (2011).
Bear, M.F., Huber, K.M. & Warren, S.T. Trends Neurosci. 27, 370–377 (2004).
Dölen, G. et al. Neuron 56, 955–962 (2007).
Bassell, G.J. & Warren, S.T. Neuron 60, 201–214 (2008).
Bhattacharya, A. et al. Neuron 76, 325–337 (2012).
Qin, M., Kang, J., Burlin, T.V., Jiang, C. & Smith, C.B. J. Neurosci. 25, 5087–5095 (2005).
Osterweil, E.K., Krueger, D.D., Reinhold, K. & Bear, M.F. J. Neurosci. 30, 15616–15627 (2010).
Huber, K.M., Kayser, M.S. & Bear, M.F. Science 288, 1254–1257 (2000).
Wu, L. et al. Neuron 21, 1129–1139 (1998).
Huang, Y., Yario, T.A. & Steitz, J.A. Proc. Natl. Acad. Sci. USA 101, 9666–9670 (2004).
Alarcon, J.M. et al. Learn. Mem. 11, 318–327 (2004).
Zearfoss, N.R., Alarcon, J.M., Trifilieff, P., Kandel, E. & Richter, J.D. J. Neurosci. 28, 8502–8509 (2008).
Huang, Y.S., Jung, M.Y., Sarkissian, M. & Richter, J.D. EMBO J. 21, 2139–2148 (2002).
Udagawa, T. et al. Mol. Cell 47, 253–266 (2012).
Berger-Sweeney, J., Zearfoss, N.R. & Richter, J.D. Learn. Mem. 13, 4–7 (2006).
Auerbach, B.D., Osterweil, E.K. & Bear, M.F. Nature 480, 63–68 (2011).
Shang, Y. et al. J. Neurochem. 111, 635–646 (2009).
Pfeiffer, B.E. & Huber, K.M. J. Neurosci. 27, 3120–3130 (2007).
Avgustinovich, D.F., Lipina, T.V., Bondar, N.P., Alekseyenko, O.V. & Kudryavtseva, N.N. Behav. Genet. 30, 101–109 (2000).
Moretti, P., Bouwknecht, J.A., Teague, R., Paylor, R. & Zoghbi, H.Y. Hum. Mol. Genet. 14, 205–220 (2005).
Laroche, S., Davis, S. & Jay, T.M. Hippocampus 10, 438–446 (2000).
Lynch, M.A. Physiol. Rev. 84, 87–136 (2004).
Feng, Y. et al. Mol. Cell 1, 109–118 (1997).
Richter, J.D., Wasserman, W.J. & Smith, L.D. Dev. Biol. 89, 159–167 (1982).
Santini, E. et al. Nature 493, 411–415 (2013).
Gkogkas, C.G. et al. Nature 493, 371–377 (2013).
Deacon, R.M.J. Nat. Protoc. 1, 1117–1119 (2006).
Raju, R.T. & Rao, B.S.S. Brain and Behavior 108–111 (National Institute of Mental Health and Neurosciences, Bangalore, India, 2004).
Antar, L.N., Afroz, R., Dictenberg, J.B., Carroll, R.C. & Bassell, G.J. J. Neurosci. 24, 2648–2655 (2004).
Svitkin, Y.V. & Sonenberg, N. Methods Enzymol. 429, 53–82 (2007).
Bordeleau, M.-E. et al. Nat. Chem. Biol. 2, 213–220 (2006).
Deacon, R.M.J. & Rawlins, J.N.P. Nat. Protoc. 1, 7–12 (2006).
Brito, L.S., Yamasaki, E.N., Paumgartten, F.J. & Brito, G.N. Braz. J. Med. Biol. Res. 20, 125–135 (1987).
Engin, E. & Treit, D. Behav. Pharmacol. 18, 365–374 (2007).
Ramos, A. & Mormede, P. Neurosci. Biobehav. Rev. 22, 33–57 (1998).
Baarendse, P.J.J. et al. Hippocampus 18, 11–19 (2008).
Morellini, F. & Schachner, M. Eur. J. Neurosci. 23, 1255–1268 (2006).
Morellini, F. et al. Cereb. Cortex 20, 2712–2727 (2010).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates (Academic Press, 2001).
McGrew, L.L. & Richter, J.D. EMBO J. 9, 3743–3751 (1990).
Acknowledgements
We thank N. Dawra for technical assistance, P. Lombroso (Yale University) and C. Proud (University of Southampton) for kind gifts of antibodies (STEP and eEF2, respectively), J. Pelletier (McGill University) for the kind gift of hippuristanol and members of the Richter lab for helpful discussions. T.U. and N.G.F. gratefully acknowledge fellowships from the FRAXA Research Foundation. J.A.H. was supported by US National Institutes of Health NRSA Postdoctoral Fellowship F32GM095060. This work was supported by NIH grants GM46779 and NS079415 to J.D.R. and MH086509 to S. Akbarian.
Author information
Authors and Affiliations
Contributions
T.U. and J.D.R. conceived of the initial project with much input from L.J.L. T.U., N.G.F. and M.J. designed and performed the majority of the experiments. H.K. and E.K. performed the electrophysiology experiments in Figure 1c,d and Supplementary Figure 4a,b. J.M.A. performed the electrophysiology experiments in Supplementary Figure 4c–e. S. Anilkumar and S.C. performed spine density analysis in Figure 1e,f and Supplementary Figure 5a,b. M.I. created and tested the CPEB antibody used in Supplementary Figure 2f. J.A.H. performed the bioinformatics analysis in Supplementary Table 1. V.C.N. and G.J.B. performed the immunocytochemistry analysis in Supplementary Figure 2a,b. T.U., N.G.F., M.J., K.N. and S. Akbarian contributed to the behavioral experiments in Figure 2. N.G.F. and J.D.R. wrote the manuscript. All authors contributed to interpretation and discussion of results and to editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 2–6 (PDF 6503 kb)
Supplementary Table
Supplementary Table 1 (XLSX 77 kb)
Source data
Rights and permissions
About this article
Cite this article
Udagawa, T., Farny, N., Jakovcevski, M. et al. Genetic and acute CPEB1 depletion ameliorate fragile X pathophysiology. Nat Med 19, 1473–1477 (2013). https://doi.org/10.1038/nm.3353
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3353
This article is cited by
-
CPEB and translational control by cytoplasmic polyadenylation: impact on synaptic plasticity, learning, and memory
Molecular Psychiatry (2023)
-
Deletion of Fmr1 in parvalbumin-expressing neurons results in dysregulated translation and selective behavioral deficits associated with fragile X syndrome
Molecular Autism (2022)
-
Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications
Signal Transduction and Targeted Therapy (2022)
-
The role of CPEB family proteins in the nervous system function in the norm and pathology
Cell & Bioscience (2021)
-
The molecular biology of FMRP: new insights into fragile X syndrome
Nature Reviews Neuroscience (2021)