Abstract
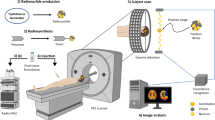
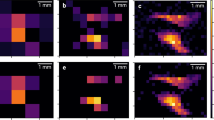
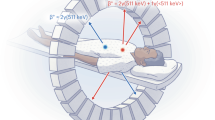
In the era of personalized medicine, there is an urgent need for in vivo techniques able to sensitively detect and quantify molecular activities. Sensitive imaging of gamma rays is widely used; however, radioactive decay is a physical constant, and its signal is independent of biological interactions. Here, we introduce a framework of previously uncharacterized targeted and activatable probes that are excited by a nuclear decay–derived signal to identify and measure molecular signatures of disease. We accomplished this by using Cerenkov luminescence, the light produced by β-particle–emitting radionuclides such as clinical positron emission tomography (PET) tracers. Disease markers were detected using nanoparticles to produce secondary Cerenkov-induced fluorescence. This approach reduces background signal compared to conventional fluorescence imaging. In addition to tumor identification from a conventional PET scan, we demonstrate the medical utility of our approach by quantitatively determining prognostically relevant enzymatic activity. This technique can be applied to monitor other markers and represents a shift toward activatable nuclear medicine agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Miyagawa, T. et al. Imaging of HSV-tk Reporter gene expression: comparison between [18F]FEAU, [18F]FFEAU, and other imaging probes. J. Nucl. Med. 49, 637–648 (2008).
Tjuvajev, J.G. et al. Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J. Nucl. Med. 43, 1072–1083 (2002).
Thorek, D.L. & Grimm, J. Enzymatically activatable diagnostic probes. Curr. Pharm. Biotechnol. 13, 523–536 (2012).
Grimm, J. et al. Use of gene expression profiling to direct in vivo molecular imaging of lung cancer. Proc. Natl. Acad. Sci. USA 102, 14404–14409 (2005).
Weissleder, R., Tung, C.H., Mahmood, U. & Bogdanov, A. Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 17, 375–378 (1999).
Rudin, M. & Weissleder, R. Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov. 2, 123–131 (2003).
Chuang, C.H. et al. In vivo positron emission tomography imaging of protease activity by generation of a hydrophobic product from a noninhibitory protease substrate. Clin. Cancer Res. 18, 238–247 (2012).
L'Annunziata, M.F. Radioactivity: Introduction and History 429 (Elsevier, Oxford, 2007).
Robertson, R. et al. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys. Med. Biol. 54, N355–N365 (2009).
Ruggiero, A., Holland, J.P., Lewis, J.S. & Grimm, J. Cerenkov luminescence imaging of medical isotopes. J. Nucl. Med. 51, 1123–1130 (2010).
Liu, H. et al. Molecular optical imaging with radioactive probes. PLoS ONE 5, e9470 (2010).
Dothager, R.S., Goiffon, R.J., Jackson, E., Harpstrite, S. & Piwnica-Worms, D. Cerenkov radiation energy transfer (CRET) imaging: a novel method for optical imaging of PET isotopes in biological systems. PLoS ONE 5, e13300 (2010).
Liu, H. et al. Radiation-luminescence-excited quantum dots for in vivo multiplexed optical imaging. Small 6, 1087–1091 (2010).
Jelley, J.V. Cerenkov radiation and its applications. Br. J. Appl. Phys. 6, 227 (1955).
Dijkers, E.C. et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 87, 586–592 (2010).
Turk, B.E., Huang, L.L., Piro, E.T. & Cantley, L.C. Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat. Biotechnol. 19, 661–667 (2001).
Väisänen, A., Kallioinen, M., Taskinen, P.J. & Turpeenniemi-Hujanen, T. Prognostic value of MMP-2 immunoreactive protein (72 kD type IV collagenase) in primary skin melanoma. J. Pathol. 186, 51–58 (1998).
Davidson, B. et al. MMP-2 and TIMP-2 expression correlates with poor prognosis in cervical carcinoma—a clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol. Oncol. 73, 372–382 (1999).
Määttä, M., Soini, Y., Liakka, A. & Autio-Harmainen, H. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumor progression and clinical prognosis. Clin. Cancer Res. 6, 2726–2734 (2000).
Dubertret, B., Calame, M. & Libchaber, A.J. Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat. Biotechnol. 19, 365–370 (2001).
Lee, S. et al. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew. Chem. Int. Ed. Engl. 47, 2804–2807 (2008).
Frank, I. & Tamm, I. Coherent visible radiation of fast electrons passing through matter. Compt. Rend. Dokl. Akad. Mauk. SSSR. 14, 109–114 (1937).
Xu, Y., Piston, D.W. & Johnson, C.H. A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins. Proc. Natl. Acad. Sci. USA 96, 151–156 (1999).
So, M.K., Xu, C., Loening, A.M., Gambhir, S.S. & Rao, J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat. Biotechnol. 24, 339–343 (2006).
Quon, A. & Gambhir, S.S. FDG-PET and beyond: molecular breast cancer imaging. J. Clin. Oncol. 23, 1664–1673 (2005).
Beattie, B.J. et al. Quantitative modeling of Cerenkov light production efficiency from medical radionuclides. PLoS ONE 7, e31402 (2012).
Kothapalli, S.R., Liu, H., Liao, J.C., Cheng, Z. & Gambhir, S.S. Endoscopic imaging of Cerenkov luminescence. Biomed. Opt. Express 3, 1215–1225 (2012).
Holland, J.P., Normand, G., Ruggiero, A., Lewis, J.S. & Grimm, J. Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emissions. Mol. Imaging 10, 177–186 (2011).
Holland, J.P., Sheh, Y. & Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 36, 729–739 (2009).
Holland, J.P. et al. Measuring the pharmacodynamic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using Zr-DFO-trastuzumab. PLoS ONE 5, e8859 (2010).
Cai, W. & Chen, X. Preparation of peptide-conjugated quantum dots for tumor vasculature-targeted imaging. Nat. Protoc. 3, 89–96 (2008).
Institute of Laboratory Animal Resources (U.S.). Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. in NIH publication v. (U.S. Department of Health and Human Services, Public Health Service, Bethesda, MD).
Flock, S.T., Jacques, S.L., Wilson, B.C., Star, W.M. & van Gemert, M.J. Optical properties of Intralipid: a phantom medium for light propagation studies. Lasers Surg. Med. 12, 510–519 (1992).
Rosenblum, G. et al. Structural basis for potent slow binding inhibition of human matrix metalloproteinase-2 (MMP-2). J. Biol. Chem. 278, 27009–27015 (2003).
Acknowledgements
We thank the staff of the Radiopharmacy at Memorial Sloan-Kettering Cancer Center (MSKCC). Scientific input was provided by M. McDevitt, N.V.K. Pillarsetty, P. Zanzonico, J. Holland and J. Lewis (of MSKCC, who also generously provided radiolabeled trastuzumab). D.L.J.T. and this work were supported by US National Institutes of Health (NIH) through the R25T Molecular Imaging Fellowship of the Molecular Imaging: Training for Oncology (MITO) Program (5R25CA096945-07; principal investigator H. Hricak). J.G. was supported by the US Department of Defense (PC111667), the Starr Cancer Consortium (I4-A427), NIH (1R01EB014944–01), the American Recovery and Reinvestment Act (73950) and the Louis V. Gerstner Young Investigator Award. Technical services provided by the Animal Imaging Core Facility were supported in part by grants from the NIH (R24 CA083084 and P30 CA008748). We thank R. Weissleder for suggestions and L. Cosgrave at MSKCC and N. Sela-Passwell of the Weizmann Institute of Science for their help.
Author information
Authors and Affiliations
Contributions
D.L.J.T. initiated the project, designed and performed experiments, analyzed the data and wrote the manuscript. B.J.B. assisted in quantitative modeling, and A.O. assisted with ex vivo experiments. J.G., as the principal investigator, contributed to the experimental design and manuscript preparation and initiated the project. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Figures 1–6 and Supplementary Tables 1–2 (PDF 9242 kb)
Rights and permissions
About this article
Cite this article
Thorek, D., Ogirala, A., Beattie, B. et al. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat Med 19, 1345–1350 (2013). https://doi.org/10.1038/nm.3323
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3323
This article is cited by
-
Practical Guidance for Developing Small-Molecule Optical Probes for In Vivo Imaging
Molecular Imaging and Biology (2023)
-
Prospective testing of clinical Cerenkov luminescence imaging against standard-of-care nuclear imaging for tumour location
Nature Biomedical Engineering (2022)
-
Radiopharmaceutical and Eu3+ doped gadolinium oxide nanoparticles mediated triple-excited fluorescence imaging and image-guided surgery
Journal of Nanobiotechnology (2021)
-
68Ga-PSMA Cerenkov luminescence imaging in primary prostate cancer: first-in-man series
European Journal of Nuclear Medicine and Molecular Imaging (2020)
-
A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging
Nature Communications (2019)