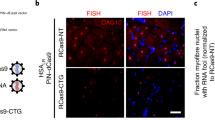
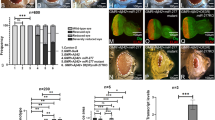
Abstract
Spinal and bulbar muscular atrophy (SBMA) is an inherited neurodegenerative disorder caused by the expansion of the polyglutamine (polyQ) tract of the androgen receptor (AR-polyQ)1,2. Characteristics of SBMA include proximal muscular atrophy, weakness, contraction fasciculation and bulbar involvement3. MicroRNAs (miRNAs) are a diverse class of highly conserved small RNA molecules that function as crucial regulators of gene expression in animals and plants4. Recent functional studies have shown the potent activity of specific miRNAs as disease modifiers both in vitro and in vivo5,6,7,8. Thus, potential therapeutic approaches that target the miRNA processing pathway have recently attracted attention9,10. Here we describe a novel therapeutic approach using the adeno-associated virus (AAV) vector–mediated delivery of a specific miRNA for SBMA. We found that miR-196a enhanced the decay of the AR mRNA by silencing CUGBP, Elav-like family member 2 (CELF2). CELF2 directly acted on AR mRNA and enhanced the stability of AR mRNA. Furthermore, we found that the early intervention of miR-196a delivered by an AAV vector ameliorated the SBMA phenotypes in a mouse model. Our results establish the proof of principle that disease-specific miRNA delivery could be useful in neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
La Spada, A.R., Wilson, E.M., Lubahn, D.B., Harding, A.E. & Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
Kennedy, W.R., Alter, M. & Sung, J.H. Progressive proximal spinal and bulbar muscular atrophy of late onset: a sex-linked recessive trait. Neurology 50, 583–593 (1998).
Sobue, G. et al. X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain 112, 209–232 (1989).
Kim, V.N., Han, J. & Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 10, 126–139 (2009).
Kim, J. et al. A MicroRNA feedback circuit in midbrain dopamine neurons. Science 317, 1220–1224 (2007).
Karres, J.S., Hilgers, V., Carrera, I., Treisman, J. & Cohen, S.M. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell 131, 136–145 (2007).
Lee, Y. et al. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat. Neurosci. 11, 1137–1139 (2008).
Williams, A.H. et al. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 326, 1549–1554 (2009).
Roshan, R., Ghosh, T., Scaria, V. & Pillai, B. MicroRNAs: novel therapeutic targets in neurodegenerative diseases. Drug Discov. Today 14, 1123–1129 (2009).
Boudreau, R.L., Rodríguez-Lebrón, E. & Davidson, B.L. RNAi medicine for the brain: progresses and challenges. Hum. Mol. Genet. 20, R21–R27 (2011).
Katsuno, M. et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854 (2002).
Katsuno, M. et al. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat. Med. 9, 768–773 (2003).
Adachi, H. et al. Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J. Neurosci. 23, 2203–2211 (2003).
Waza, M. et al. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat. Med. 11, 1088–1095 (2005).
Tokui, K. et al. 17-DMAG ameliorates polyglutamine-mediated motor neuron degeneration through well-preserved proteasome function in an SBMA model mouse. Hum. Mol. Genet. 18, 898–910 (2009).
Eacker, S.M., Dawson, T.M. & Dawson, V.L. Understanding microRNAs in neurodegeneration. Nat. Rev. Neurosci. 10, 837–841 (2009).
Bilen, J., Liu, N., Burnett, B.G., Pittman, R.N. & Bonini, N.M. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol. Cell 24, 157–163 (2006).
Schaefer, A. et al. Cerebellar neurodegeneration in the absence of microRNAs. J. Exp. Med. 204, 1553–1558 (2007).
Kaspar, B.K. et al. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301, 839–842 (2003).
Towne, C. et al. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 17, 141–146 (2010).
Dominguez, E. et al. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum. Mol. Genet. 20, 681–693 (2011).
Fu, H., Dirosario, J., Killedar, S., Zaraspe, K. & McCarty, D.M. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol. Ther. 19, 1025–1033 (2011).
Petrs-Silva, H. et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol. Ther. 19, 293–301 (2011).
Hui, A.B. et al. Robust global micro-RNA profiling with formalin-fixed paraffin-embedded breast cancer tissues. Lab. Invest. 89, 597–606 (2009).
Maru, D.M. et al. MicroRNA-196a is a potential marker of progression during Barrett's metaplasia-dysplasia-invasive adenocarcinoma sequence in esophagus. Am. J. Pathol. 174, 1940–1948 (2009).
Yekta, S., Shih, I.H. & Bartel, D.P. MicroRNA-directed cleavage of HOXB8 mRNA. Science 304, 594–596 (2004).
Hornstein, E. et al. The microRNA miR-196 acts upstream of Hoxb8 and Shh in limb development. Nature 438, 671–674 (2005).
Pedersen, I.M. et al. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature 449, 919–922 (2007).
Dhaenens, C.M. et al. Mis-splicing of Tau exon 10 in myotonic dystrophy type 1 is reproduced by overexpression of CELF2 but not by MBNL1 silencing. Biochim. Biophys. Acta 1812, 732–742 (2011).
Natarajan, G. et al. CUGBP2 downregulation by prostaglandin E2 protects colon cancer cells from radiation-induced mitotic catastrophe. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1235–G1244 (2008).
Subramaniam, D. et al. RNA binding protein CUGBP2/CELF2 mediates curcumin-induced mitotic catastrophe of pancreatic cancer cells. PLoS ONE 6, e16958 (2011).
Kobayashi, Y. et al. Chaperones Hsp70 and Hsp40 suppress aggregate formation and apoptosis in cultured neuronal cells expressing truncated androgen receptor protein with expanded polyglutamine tract. J. Biol. Chem. 275, 8772–8778 (2000).
Katsuno, M. et al. Disrupted transforming growth factor-β signaling in spinal and bulbar muscular atrophy. J. Neurosci. 30, 5702–5712 (2010).
Gao, G. et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78, 6381–6388 (2004).
Li, X.G. et al. Viral-mediated temporally controlled dopamine production in a rat model of Parkinson disease. Mol. Ther. 13, 160–166 (2006).
Acknowledgements
We are grateful to T. Cooper (Department of Pathology and Immunology, Baylor College of Medicine) for kindly providing the plasmid expressing human CELF2. We also thank N. Takino, H. Miyauchi and K. Ayabe (Division of Neurology, Department of Medicine, Jichi Medical University) for their help with the production of the AAV vectors and Y. Kondo and K. Shinjo (Division of Molecular Oncology, Aichi Cancer Center Research Institute) for expert technical support and helpful discussions. This work was supported by grants from the Ministry of Health, Labor and Welfare; grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a Center-of-Excellence (COE) grant; a Grant-in-Aid for Scientific Research on Innovated Areas “Foundations of Synapse and Neurocircuit Pathology”; and the Core Research for Evolutional Science and Technology (CREST) program of the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Contributions
Project planning was done by Y.M., H.A., M.K., F.T., S. Muramatsu and G.S.; experimental work was done by Y.M., H.A., M.K., M.M., Y.-M.J., Z.H., H.D., S. Matsumoto, N.K., M.I., G.T. and S. Muramatsu; data analysis was done by Y.M., H.A., M.K., F.T., S. Muramatsu and G.S.; the manuscript was written by Y.M., H.A., M.K., F.T., S. Muramatsu and G.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Note and Supplementary Figures 1–10 (PDF 7641 kb)
Rights and permissions
About this article
Cite this article
Miyazaki, Y., Adachi, H., Katsuno, M. et al. Viral delivery of miR-196a ameliorates the SBMA phenotype via the silencing of CELF2. Nat Med 18, 1136–1141 (2012). https://doi.org/10.1038/nm.2791
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2791
This article is cited by
-
MiR-138 is a potent regulator of the heterogenous MYC transcript population in cancers
Oncogene (2022)
-
RETRACTED ARTICLE: GAS5 knockdown alleviates spinal cord injury by reducing VAV1 expression via RNA binding protein CELF2
Scientific Reports (2021)
-
Recent progress in microRNA-based delivery systems for the treatment of human disease
ExRNA (2019)
-
Differential expression of microRNAs and other small RNAs in muscle tissue of patients with ALS and healthy age-matched controls
Scientific Reports (2018)
-
In Silico Mining of Conserved miRNAs of Indian Catfish Clarias batrachus (Linnaeus, 1758) from the Contigs, ESTs, and BAC End Sequences
Applied Biochemistry and Biotechnology (2017)