Abstract
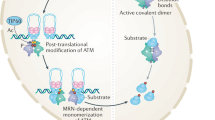
Ataxia telangiectasia is a neurodegenerative disease caused by mutation of the Atm gene. Here we report that ataxia telangiectasia mutated (ATM) deficiency causes nuclear accumulation of histone deacetylase 4 (HDAC4) in neurons and promotes neurodegeneration. Nuclear HDAC4 binds to chromatin, as well as to myocyte enhancer factor 2A (MEF2A) and cAMP-responsive element binding protein (CREB), leading to histone deacetylation and altered neuronal gene expression. Blocking either HDAC4 activity or its nuclear accumulation blunts these neurodegenerative changes and rescues several behavioral abnormalities of ATM-deficient mice. Full rescue of the neurodegeneration, however, also requires the presence of HDAC4 in the cytoplasm, suggesting that the ataxia telangiectasia phenotype results both from a loss of cytoplasmic HDAC4 as well as its nuclear accumulation. To remain cytoplasmic, HDAC4 must be phosphorylated. The activity of the HDAC4 phosphatase, protein phosphatase 2A (PP2A), is downregulated by ATM-mediated phosphorylation. In ATM deficiency, enhanced PP2A activity leads to HDAC4 dephosphorylation and the nuclear accumulation of HDAC4. Our results define a crucial role of the cellular localization of HDAC4 in the events leading to ataxia telangiectasia neurodegeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Barzilai, A., Biton, S. & Shiloh, Y. The role of the DNA damage response in neuronal development, organization and maintenance. DNA Repair (Amst.) 7, 1010–1027 (2008).
Bundey, S. Clinical and genetic features of ataxia-telangiectasia. Int. J. Radiat. Biol. 66, S23–S29 (1994).
Lavin, M.F. & Shiloh, Y. The genetic defect in ataxia-telangiectasia. Annu. Rev. Immunol. 15, 177–202 (1997).
Savitsky, K. et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268, 1749–1753 (1995).
Sedgwick, R.P. & Boder, E. Progressive ataxia in childhood with particular reference to ataxia-telangiectasia. Neurology 10, 705–715 (1960).
D'Mello, S.R. Histone deacetylases as targets for the treatment of human neurodegenerative diseases. Drug News Perspect. 22, 513–524 (2009).
Grozinger, C.M. & Schreiber, S.L. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14–3-3–dependent cellular localization. Proc. Natl. Acad. Sci. USA 97, 7835–7840 (2000).
Haberland, M., Montgomery, R.L. & Olson, E.N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 10, 32–42 (2009).
Majdzadeh, N., Morrison, B.E. & D'Mello, S.R. Class IIA HDACs in the regulation of neurodegeneration. Front. Biosci. 13, 1072–1082 (2008).
Yang, X.J. & Seto, E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 9, 206–218 (2008).
Darcy, M.J., Calvin, K., Cavnar, K. & Ouimet, C.C. Regional and subcellular distribution of HDAC4 in mouse brain. J. Comp. Neurol. 518, 722–740 (2010).
Grozinger, C.M., Hassig, C.A. & Schreiber, S.L. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. USA 96, 4868–4873 (1999).
Wang, A.H. et al. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol. Cell. Biol. 19, 7816–7827 (1999).
Majdzadeh, N. et al. HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev. Neurobiol. 68, 1076–1092 (2008).
McKinsey, T.A., Zhang, C.L., Lu, J. & Olson, E.N. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408, 106–111 (2000).
Wang, A.H. et al. Regulation of histone deacetylase 4 by binding of 14–3-3 proteins. Mol. Cell. Biol. 20, 6904–6912 (2000).
Zhao, X. et al. The modular nature of histone deacetylase HDAC4 confers phosphorylation-dependent intracellular trafficking. J. Biol. Chem. 276, 35042–35048 (2001).
Bolger, T.A. & Yao, T.P. Intracellular trafficking of histone deacetylase 4 regulates neuronal cell death. J. Neurosci. 25, 9544–9553 (2005).
Chen, B. & Cepko, C.L. HDAC4 regulates neuronal survival in normal and diseased retinas. Science 323, 256–259 (2009).
Youn, H.D., Grozinger, C.M. & Liu, J.O. Calcium regulates transcriptional repression of myocyte enhancer factor 2 by histone deacetylase 4. J. Biol. Chem. 275, 22563–22567 (2000).
Kuljis, R.O., Xu, Y., Aguila, M.C. & Baltimore, D. Degeneration of neurons, synapses, and neuropil and glial activation in a murine Atm knockout model of ataxia-telangiectasia. Proc. Natl. Acad. Sci. USA 94, 12688–12693 (1997).
Xu, Y. et al. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 10, 2411–2422 (1996).
Backs, J., Backs, T., Bezprozvannaya, S., McKinsey, T.A. & Olson, E.N. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol. Cell. Biol. 28, 3437–3445 (2008).
Méjat, A. et al. Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat. Neurosci. 8, 313–321 (2005).
Korzus, E., Rosenfeld, M.G. & Mayford, M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42, 961–972 (2004).
Nervi, C. et al. Inhibition of histone deacetylase activity by trichostatin A modulates gene expression during mouse embryogenesis without apparent toxicity. Cancer Res. 61, 1247–1249 (2001).
Yoshida, M., Kijima, M., Akita, M. & Beppu, T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265, 17174–17179 (1990).
Barlow, C. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171 (1996).
Liu, Y., Randall, W.R. & Schneider, M.F. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J. Cell Biol. 168, 887–897 (2005).
Paroni, G. et al. PP2A regulates HDAC4 nuclear import. Mol. Biol. Cell 19, 655–667 (2008).
Shi, Y. Serine/threonine phosphatases: mechanism through structure. Cell 139, 468–484 (2009).
Matsuoka, S. et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160–1166 (2007).
Chan, J.K., Sun, L., Yang, X.J., Zhu, G. & Wu, Z. Functional characterization of an amino-terminal region of HDAC4 that possesses MEF2 binding and transcriptional repressive activity. J. Biol. Chem. 278, 23515–23521 (2003).
Miska, E.A. et al. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 18, 5099–5107 (1999).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Li, H. et al. The Sequence Alignment/Map (SAM) format and SAMtools. Bioinformatics. 25, 2078–2079 (2009).
Fejes, A.P. et al. FindPeaks 3.1: a tool for identifying areas of enrichment from massively parallel short-read sequencing technology. Bioinformatics 24, 1729–1730 (2008).
Kaemmerer, W.F. et al. In vivo transduction of cerebellar Purkinje cells using adeno-associated virus vectors. Mol. Ther. 2, 446–457 (2000).
Cortés, M.L., Oehmig, A., Perry, K.F., Sanford, J.D. & Breakefield, X.O. Expression of human ATM cDNA in Atm-deficient mouse brain mediated by HSV-1 amplicon vector. Neuroscience 141, 1247–1256 (2006).
Cooper, J.F. & Kusnecov, A.W. Methylmercuric chloride induces activation of neuronal stress circuitry and alters exploratory behavior in the mouse. Neuroscience 148, 1048–1064 (2007).
Acknowledgements
We thank Y. Xu (University of California San Diego) for providing homozygous mice carrying the Atm knockout mutation, tm1Bal. We thank R. Gatti (University of California Los Angeles) for sharing human ataxia telangiectasia paraffin-fixed samples. Human frozen tissue was obtained from the National Institute of Child Health and Human Development (NICHD) Brain and Tissue Bank for Developmental Disorders (NICHD contracts N01-HD-4-3368 and N01-HD-4-3383). We thank M.B. Kastan (Duke University), T. Yao (Duke University) and S. Schreiber (Harvard University) for providing the wild-type and kinase-dead Flag-ATM, GFP-HDAC4 and Flag-HADC4 plasmids. M. Swerdel prepared the SOLiD ChIP-seq libraries. The long-term support of the Ataxia Telangiectasia Children's Project to K.H. is gratefully acknowledged. This work was also supported by grants from the US National Institutes of Health (NS20591 and NS71022) to K.H., R.P.H. (R21 MH085088) and A.K. (MH60706 and NIEHS P30ES05022). C.L.R. is a National Science Foundation Integrative Graduate Education and Research Traineeship fellow.
Author information
Authors and Affiliations
Contributions
J.L. and K.H. designed the experiments, analyzed data and wrote the manuscript. C.L.R. and R.P.H. developed, carried out and analyzed data for the ChIP-seq analyses. J.L. and J.C. carried out the immunocytochemistry experiments. J.C. performed all of the qPCR experiments. J.L. and A.K. carried out the mouse cerebellar lentiviral injections. M.S.S., J.L. and A.K. carried out behavioral tests.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–3 and Supplementary Methods (PDF 2592 kb)
Rights and permissions
About this article
Cite this article
Li, J., Chen, J., Ricupero, C. et al. Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia telangiectasia. Nat Med 18, 783–790 (2012). https://doi.org/10.1038/nm.2709
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2709
This article is cited by
-
Deciphering the roles of subcellular distribution and interactions involving the MEF2 binding region, the ankyrin repeat binding motif and the catalytic site of HDAC4 in Drosophila neuronal morphogenesis
BMC Biology (2024)
-
Disproportionate Expression of ATM in Cerebellar Cortex During Human Neurodevelopment
The Cerebellum (2023)
-
The phosphorylation to acetylation/methylation cascade in transcriptional regulation: how kinases regulate transcriptional activities of DNA/histone-modifying enzymes
Cell & Bioscience (2022)
-
A signalling pathway for transcriptional regulation of sleep amount in mice
Nature (2022)
-
Organic anion transporter 1 is an HDAC4-regulated mediator of nociceptive hypersensitivity in mice
Nature Communications (2022)