Abstract
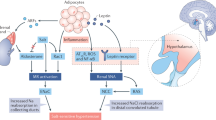
How high salt intake increases blood pressure is a key question in the study of hypertension. Salt intake induces increased renal sympathetic activity resulting in sodium retention. However, the mechanisms underlying the sympathetic control of renal sodium excretion remain unclear. In this study, we found that β2-adrenergic receptor (β2AR) stimulation led to decreased transcription of the gene encoding WNK4, a regulator of sodium reabsorption. β2AR stimulation resulted in cyclic AMP-dependent inhibition of histone deacetylase-8 (HDAC8) activity and increased histone acetylation, leading to binding of the glucocorticoid receptor to a negative glucocorticoid−responsive element in the promoter region. In rat models of salt-sensitive hypertension and sympathetic overactivity, salt loading suppressed renal WNK4 expression, activated the Na+-Cl− cotransporter and induced salt-dependent hypertension. These findings implicate the epigenetic modulation of WNK4 transcription in the development of salt-sensitive hypertension. The renal β2AR-WNK4 pathway may be a therapeutic target for salt-sensitive hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
04 August 2011
In the version of this article initially published, the authors made several inadvertent errors during manuscript preparation. In Figure 3f the trace for WT mice was incorrect, and in Figure 4a the bands shown for 'Total GR' were incorrect. These errors did not affect the quantification of band intensities shown in Figure 4a and did not affect any of the conclusions of the article. The errors have been corrected in the HTML and PDF versions of the article.
05 April 2012
In the version of this article initially published, the image of the actin bands shown in Supplementary Figure 2b was mistakenly rotated 180 degrees. The image has been replaced with the bands in their correct orientation, and the densitometry shown for this blot has been recalculated, which does not affect the conclusions. The figure legends for Figure 1e and Supplementary Figure 2b have also been edited to indicate that the same kidney samples were used for the blots in Figure 1b,e and Supplementary Figure 2b and that the actin bands shown for these blots are identical.
References
Iwamoto, T. et al. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat. Med. 10, 1193–1199 (2004).
Shibata, S. et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat. Med. 14, 1370–1376 (2008).
Machnik, A. al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C–dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Fujita, T., Henry, W.L., Bartter, F.C., Lake, C.R. & Delea, C.S. Factors influencing blood pressure in salt-sensitive patients with hypertension. Am. J. Med. 69, 334–344 (1980).
Campese, V.M. et al. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt-sensitive patients with essential hypertension. Kidney Int. 21, 371–378 (1982).
Gill, J.R., Gullner, G.R. Jr., Lake, C.R., Lakatua, D.J. & Lan, G. Plasma and urinary catecholamines in salt-sensitive idiopathic hypertension. Hypertension 11, 312–319 (1988).
Ono, A., Kuwaki, T., Kumada, M. & Fujita, T. Differential central modulation of the baroreflex by salt loading in normotensive and spontaneously hypertensive rats. Hypertension 29, 808–814 (1997).
Fujita, T. & Sato, Y. Role of hypothalamic-renal noradrenergic systems in hypertensive action of potassium. Hypertension 20, 466–472 (1992).
Katholi, R.E., Naftilan, A.J. & Oparil, S. Importance of renal sympathetic tone in the development of DOCA-salt hypertension in the rat. Hypertension 2, 266–273 (1980).
Lohmeier, T.E., Tillman, L.J., Carroll, R.G., Brown, A.J. & Guyton, A. Malignant hypertensive crisis induced by chronic intrarenal norepinephrine infusion. Hypertension 6, I177–I182 (1984).
DiBona, G.F. Physiology in perspective: the wisdom of the body. Neural control of the kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R633–R641 (2005).
Guild, S.J. et al. Regional responsiveness of renal perfusion to activation of the renal nerves. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R1177–R1186 (2002).
Kahle, K.T., Ring, A.M. & Lifton, R.P. Molecular physiology of the WNK kinases. Annu. Rev. Physiol. 70, 329–355 (2008).
Wilson, F.H. et al. Human hypertension caused by mutations in WNK kinases. Science 293, 1107–1112 (2001).
Yang, C.L., Zhu, X. & Ellison, D.H. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J. Clin. Invest. 117, 3403–3411 (2007).
Ring, A.M. et al. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc. Natl. Acad. Sci. USA 104, 4020–4024 (2007).
Lalioti, M.D. et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat. Genet. 38, 1124–1132 (2006).
O'Reilly, M. et al. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J. Am. Soc. Nephrol. 17, 2402–2413 (2006).
Chiga, M. et al. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int. 74, 1403–1409 (2008).
Li, J. & Wang, D. Function and regulation of epithelial sodium transporters in the kidney of a salt-sensitive hypertensive rat model. J. Hypertens. 25, 1065–1072 (2007).
Vasquez, M.M. et al. Induction of serum- and glucocorticoid-induced kinase-1 (SGK1) by cAMP regulates increases in α-ENaC. J. Cell. Physiol. 217, 632–642 (2008).
Willyard, C. The saving switch. Nat. Med. 16, 18–21 (2010).
Bechtel, W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550 (2010).
Wurtele, H. et al. Modulation of histone H3 lysine 56 acetylation as an antifungal therapeutic strategy. Nat. Med. 16, 774–780 (2010).
Villagra, A., Sotomayor, E.M. & Seto, E. Histone deacetylases and the immunological network: implications in cancer and inflammation. Oncogene 29, 157–173 (2010).
Marumo, T., Hishikawa, K., Yoshikawa, M. & Fujita, T. Epigenetic regulation of BMP7 in the regenerative response to ischemia. J. Am. Soc. Nephrol. 19, 1311–1320 (2008).
Peleg, S. et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328, 753–756 (2010).
Mulholland, N.M., Snyder, S.K., Kolla, S.S. & Smith, C.L. Chromatin-dependent regulation of the MMTV promoter by cAMP signaling is mediated through distinct pathways. Exp. Cell Res. 287, 361–373 (2003).
Lee, H., Rezai-Zadeh, N. & Seto, E. Negative regulation of histone deacetylase 8 activity by cyclic AMP-dependent protein kinase A. Mol. Cell. Biol. 24, 765–773 (2004).
Guyton, A.C. Blood pressure control—special role of the kidney and body fluids. Science 252, 1813–1816 (1991).
Rozansky, D.J. et al. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J. Clin. Invest. 119, 2601–2612 (2009).
San-Cristobal, P. et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK–dependent pathway. Proc. Natl. Acad. Sci. USA 106, 4384–4389 (2009).
Nguyen Dinh Cat, A. et al. Conditional transgenic mice for studying the role of the glucocorticoid receptor in the renal collecting duct. Endocrinology 150, 2202–2210 (2009).
Rangarajan, P.N., Umesosno, K. & Evans, M. Modulation of glucocorticoid receptor function by protein kinase A. Mol. Endocrinol. 6, 1451–1457 (1992).
Massaad, C., Houard, N., Lombes, M. & Barouki, R. Modulation of human mineralocorticoid receptor function by protein kinase A. Mol. Endocrinol. 13, 57–65 (1999).
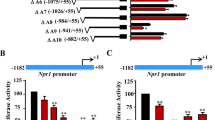
Li, C. et al. Glucocorticoid repression of human with-no-lysine (K) kinase-4 gene expression is mediated by the negative response elements in the promoter. J. Mol. Endocrinol. 40, 3–12 (2008).
Goodwin, J.E., Zhang, J., Velazquez, H. & Geller, D.S. The glucocorticoid receptor in the distal nephron is not necessary for the development or maintenance of dexamethasone-induced hypertension. Biochem. Biophys. Res. Commun. 394, 266–271 (2010).
Astrand, C., Belikov, S. & Wrange, O. Histone acetylation characterizes chromatin presetting by NF1 and Oct1 and enhances glucocorticoid receptor binding to the MMTV promoter. Exp. Cell Res. 315, 2604–2615 (2009).
Isohashi, F. et al. Interaction of histones in glucocorticoid receptor binding to DNA in vitro. Cancer Res. 49, 2235s–2237s (1989).
Huang, B.S., Wang, H. & Leenen, F.H. Enhanced sympathoexcitatory and pressor responses to central Na+ in central salt-sensitive vs. -resistant rats. Am. J. Physiol. Heart. Circ. Physiol. 281, H1881–H1889 (2001).
Adams, J.M., Madden, C.J., Sved, A.F. & Stocker, S.D. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension 50, 354–359 (2007).
Greven, J., Heidenreich, O., Greven, U. & Hildebrand, S. A micropuncture study of the effect of isoprenaline on renal tubular fluid and electrolyte transport in the rat. Naunyn Schmiedebergs Arch. Pharmacol. 287, 117–128 (1975).
Meier, K.E., Snavely, M.D., Brown, S.L., Brown, J.H. & Insel, P.A. α1- and β2-adrenergic receptor expression in the Madin-Darby canine kidney epithelial cell line. J. Cell Biol. 97, 405–415 (1983).
Barajas, L., Powers, K.V. & Wang, P. Innervation of the renal cortical tubules: a quantitative study. Am. J. Physiol. 247, F50–F60 (1984).
King, B.R., Smith, R. & Nicholson, R.C. Novel glucocorticoid and cAMP interactions on the CRH gene promoter. Mol. Cell. Endocrinol. 194, 19–28 (2002).
Leader, J.E., Wang, C., Fu, M. & Pestell, R.G. Epigenetic regulation of nuclear steroid receptors. Biochem. Pharmacol. 72, 1589–1596 (2006).
Li, B., Carey, M. & Workman, J.L. The role of chromatin during transcription. Cell 128, 707–719 (2007).
Snyder, E.M., Turner, S.T., Joyner, M.J., Eisenach, J.H. & Johnson, B.D. The Arg16Gly polymorphism of the β2-adrenergic receptor and the natriuretic response to rapid saline infusion in humans. J. Physiol. (Lond.) 574, 947–954 (2006).
Pojoga, L. et al. β-2 adrenergic receptor diplotype defines a subset of salt-sensitive hypertension. Hypertension 48, 892–900 (2006).
Koepke, J.P., Jones, S. & DiBona, G.F. Hypothalamic β2-adrenoceptor control of renal sympathetic nerve activity and urinary sodium excretion in conscious, spontaneously hypertensive rats. Circ. Res. 58, 241–248 (1986).
Fujita, T. & Sato, Y. Hypotensive effect of taurine: possible involvement of the sympathetic nervous system and endogenous opiates. J. Clin. Invest. 82, 993–997 (1988).
Nagase, M. et al. Podocyte injury underlies the glomerulopathy of Dahl salt-sensitive rats and is reversed by aldosterone blocker. Hypertension 47, 1084–1093 (2006).
Hait, N.C. et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 (2009).
Acknowledgements
We thank K. Tamura (Yokohama City Medical University) for providing mDCT cells. Antibodies targeting NCC and p-NCC were provided by D.H. Ellison (Oregon Health and Science University) and S. Uchida (Tokyo Dental and Medical University), respectively. We also thank G. McMahon for his contribution to data discussion. Eplerenone and olmesartan were provided by Pfizer and Daiichi-Sankyo, respectively. This work was supported by research grants from the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (T.F.).
Author information
Authors and Affiliations
Contributions
S.Y.M. carried out both in vitro and in vivo experiments and wrote the manuscript during a PhD course under the direction of T.F. at the University of Tokyo; T.S. carried out in vivo experiments and conducted experiments; S.O., H.W., Y.U., F.K.-M., Y.Y. and T.M. helped with experimental procedures and contributed to data discussion; D.S.G. generated distal nephron-specific glucocorticoid receptor–knockout mice and H.T. provided glucocorticoid receptor plasmids and contributed to data discussion. T.F. designed and directed the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18 and Supplementary Table 1 (PDF 820 kb)
Rights and permissions
About this article
Cite this article
Mu, S., Shimosawa, T., Ogura, S. et al. Epigenetic modulation of the renal β-adrenergic–WNK4 pathway in salt-sensitive hypertension. Nat Med 17, 573–580 (2011). https://doi.org/10.1038/nm.2337
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2337
This article is cited by
-
Role of glucocorticoid receptor mutations in hypertension and adrenal gland hyperplasia
Pflügers Archiv - European Journal of Physiology (2022)
-
Kidney and epigenetic mechanisms of salt-sensitive hypertension
Nature Reviews Nephrology (2021)
-
Lysine acetyltransferases and lysine deacetylases as targets for cardiovascular disease
Nature Reviews Cardiology (2020)
-
Maternal elevated salt consumption and the development of autism spectrum disorder in the offspring
Journal of Neuroinflammation (2019)
-
Azilsartan causes natriuresis due to its sympatholytic action in kidney disease
Hypertension Research (2019)