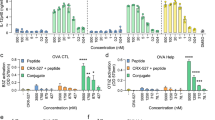
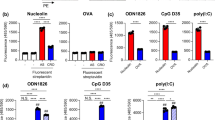
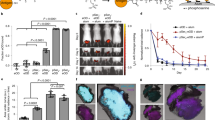
Abstract
As an approved vaccine adjuvant for use in humans, alum has vast health implications, but, as it is a crystal, questions remain regarding its mechanism. Furthermore, little is known about the target cells, receptors, and signaling pathways engaged by alum. Here we report that, independent of inflammasome and membrane proteins, alum binds dendritic cell (DC) plasma membrane lipids with substantial force. Subsequent lipid sorting activates an abortive phagocytic response that leads to antigen uptake. Such activated DCs, without further association with alum, show high affinity and stable binding with CD4+ T cells via the adhesion molecules intercellular adhesion molecule-1 (ICAM-1) and lymphocyte function–associated antigen-1 (LFA-1). We propose that alum triggers DC responses by altering membrane lipid structures. This study therefore suggests an unexpected mechanism for how this crystalline structure interacts with the immune system and how the DC plasma membrane may behave as a general sensor for solid structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Glenny, A.T., Pope, C.G., Waddington, H. & Wallace, V. The antigenic value of toxoid precipitated by potassium alum. Receptors control activation of adaptive immune responses. J. Pathol. Bacteriol. 29, 38–45 (1926).
Parham, P. The Immune System 2nd ed. Ch. 12 (Garland Science, 2004).
Marrack, P., McKee, A.S. & Munks, M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 9, 287–293 (2009).
Glenny, A.T., Buttle, G.A.H. & Stevens, M.F. Rate of disappearance of diphtheria toxoid injected into rabbits and guinea-pigs: toxoid precipitated with alum. J. Pathol. Bacteriol. 34, 267–275 (1931).
Jordan, M.B., Mills, D.M., Kappler, J., Marrack, P. & Cambier, J.C. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science 304, 1808–1810 (2004).
Kool, M. et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 205, 869–882 (2008).
Eisenbarth, S.C., Colegio, O.R., O'Connor, W., Sutterwala, F.S. & Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 (2008).
Sharp, F.A. et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. USA 106, 870–875 (2009).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Franchi, L. & Nunez, G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 38, 2085–2089 (2008).
Kool, M. et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 181, 3755–3759 (2008).
McKee, A.S. et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 183, 4403–4414 (2009).
Leonenko, Z., Finot, E. & Amrein, M. Adhesive interaction measured between AFM probe and lung epithelial type II cells. Ultramicroscopy 107, 948–953 (2007).
Ng, G. et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 29, 807–818 (2008).
Harlow, E. & Lane, D. Antibodies—a Laboratory Manual Ch. 5 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1988).
Coligan, J.E. Short Protocols in Immunology Ch. 1 (Wiley, 2005).
Goldstein, J. et al. Scanning Electron Microscopy and X-ray Microanalysis Ch. 7 (Springer, 2003).
Garry, V.F., Good, P.F., Manivel, J.C. & Perl, D.P. Investigation of a fatality from nonoccupational aluminum phosphide exposure: measurement of aluminum in tissue and body fluids as a marker of exposure. J. Lab. Clin. Med. 122, 739–747 (1993).
Lanier, L.L., Corliss, B.C., Wu, J., Leong, C. & Phillips, J.H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703–707 (1998).
Crowley, M.T. et al. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J. Exp. Med. 186, 1027–1039 (1997).
Fitzer-Attas, C.J. et al. Fcγ receptor–mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr and Lyn. J. Exp. Med. 191, 669–682 (2000).
Jiang, K. et al. Syk regulation of phosphoinositide 3-kinase–dependent NK cell function. J. Immunol. 168, 3155–3164 (2002).
Dai, R., Chen, R. & Li, H. Cross-talk between PI3K/Akt and MEK/ERK pathways mediates endoplasmic reticulum stress-induced cell cycle progression and cell death in human hepatocellular carcinoma cells. Int. J. Oncol. 34, 1749–1757 (2009).
Rommel, C. et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286, 1738–1741 (1999).
Shiratsuchi, H. & Basson, M.D. Extracellular pressure stimulates macrophage phagocytosis by inhibiting a pathway involving FAK and ERK. Am. J. Physiol. Cell Physiol. 286, C1358–C1366 (2004).
Kovjazin, R. et al. Ferrocene-induced lymphocyte activation and anti-tumor activity is mediated by redox-sensitive signaling. FASEB J. 17, 467–469 (2003).
Mathur, R.K., Awasthi, A., Wadhone, P., Ramanamurthy, B. & Saha, B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat. Med. 10, 540–544 (2004).
Newton, R. et al. The MAP kinase inhibitors, PD098059, UO126 and SB203580, inhibit IL-1β–dependent PGE2 release via mechanistically distinct processes. Br. J. Pharmacol. 130, 1353–1361 (2000).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Greenberg, S. & Grinstein, S. Phagocytosis and innate immunity. Curr. Opin. Immunol. 14, 136–145 (2002).
Yeung, T. et al. Receptor activation alters inner surface potential during phagocytosis. Science 313, 347–351 (2006).
Sun, H., Pollock, K.G. & Brewer, J.M. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine 21, 849–855 (2003).
Rock, K.L. & Shen, L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol. Rev. 207, 166–183 (2005).
Rock, K.L. Exiting the outside world for cross-presentation. Immunity 25, 523–525 (2006).
Shi, Y., Evans, J.E. & Rock, K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521 (2003).
Shi, Y., Galusha, S.A. & Rock, K.L. Cutting edge: elimination of an endogenous adjuvant reduces the activation of CD8 T lymphocytes to transplanted cells and in an autoimmune diabetes model. J. Immunol. 176, 3905–3908 (2006).
Kovacsovics-Bankowski, M. & Rock, K.L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science 267, 243–246 (1995).
Rimaniol, A.C. et al. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine 22, 3127–3135 (2004).
Martinon, F. Detection of immune danger signals by NALP3. J. Leukoc. Biol. 83, 507–511 (2008).
Chen, C.J. et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J. Clin. Invest. 116, 2262–2271 (2006).
Gross, O. et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459, 433–436 (2009).
Ruland, J. CARD9 signaling in the innate immune response. Ann. NY Acad. Sci. 1143, 35–44 (2008).
Shio, M.T. et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 5, e1000559 (2009).
Shi, Y., Mucsi, A.D. & Ng, G. Monosodium urate crystals in inflammation and immunity. Immunol. Rev. 233, 203–217 (2010).
De Gregorio, E., D'Oro, U. & Wack, A. Immunology of TLR-independent vaccine adjuvants. Curr. Opin. Immunol. 21, 339–345 (2009).
Kanevets, U., Sharma, K., Dresser, K. & Shi, Y. A role of IgM antibodies in monosodium urate crystal formation and associated adjuvanticity. J. Immunol. 182, 1912–1918 (2009).
Richter, R., Mukhopadhyay, A. & Brisson, A. Pathways of lipid vesicle deposition on solid surfaces: a combined QCM-D and AFM study. Biophys. J. 85, 3035–3047 (2003).
Nollert, P., Kiefer, H. & Jahnig, F. Lipid vesicle adsorption versus formation of planar bilayers on solid surfaces. Biophys. J. 69, 1447–1455 (1995).
Desrosiers, M.D. et al. Adenosine deamination sustains dendritic cell activation in inflammation. J. Immunol. 179, 1884–1892 (2007).
Acknowledgements
We thank L. Lanier and C. Lowell (University of California–San Francisco) for providing mouse bone marrows; P. Kubes (University of Calgary) for ICAM-1–, LFA-1– and TLR4-knockout mice; K. Rock (University of Massachusetts Medical School), P. Cresswell (Yale University), G. Dranoff (Harvard Medical School) and R. Yates and J. Deans (University of Calgary) for cell lines, L.J. Shen, M. Ho, Y. Yang and P. Santamaria for manuscript review; C. Olson and T. Fung for statistical software and advice; E. Chau for manuscript editing; F. Oskouie for cell culture assistance; and M. Schoel and W.-D. Xiang for SEM and transmission electron microscopy technical assistance. This work was supported by grants from the US National Institutes of Health to Y.S. and equipment donation from JPK instruments to M.W.A.
Author information
Authors and Affiliations
Contributions
Y.S. designed experiments with input from M.W.A. and wrote the manuscript with assistance from T.L.F. and D.A.M. T.L.F. performed the experiments unless indicated otherwise below. G.N. performed EDS and SEM assays and developed methods for lipid-crystal binding analysis. A.H. and A.D.M. performed CD4-DC binding analysis and EDS and SEM work and contributed to bilayer lipid synthesis. M.D.D. performed antibody induction and cytokine studies with assistance from Y.F. S.M.W., P.Z. and C.C.L. performed aliphatic chain extension on cholesterol and sphingomyelin. M.E.S. and A.V. performed western blotting. E.P. provided Langmuir trough and technical assistance. J.T. and D.A.M. provided inflammasome-deficient mice and technical assistance and consultation. M.W.A. supervised all aspects of work involving AFM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Methods and Supplementary Data (PDF 1381 kb)
Rights and permissions
About this article
Cite this article
Flach, T., Ng, G., Hari, A. et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med 17, 479–487 (2011). https://doi.org/10.1038/nm.2306
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2306
This article is cited by
-
An inventory of adjuvants used for vaccination in horses: the past, the present and the future
Veterinary Research (2023)
-
Potentiating humoral and cellular immunity using a novel hybrid polymer-lipid nanoparticle adjuvant for HBsAg-VLP vaccine
Journal of Nanobiotechnology (2023)
-
Human dendritic cell maturation induced by amorphous silica nanoparticles is Syk-dependent and triggered by lipid raft aggregation
Particle and Fibre Toxicology (2023)
-
Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells
Nature (2023)
-
Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action
Nature Reviews Chemistry (2021)